Sulfide (organic) facts for kids
A sulfide is a special type of chemical compound that contains a sulfur atom connected to two other carbon-based groups. You might also hear them called thioethers. Think of them like ethers, which have an oxygen atom connecting two groups, but in sulfides, a sulfur atom takes the place of the oxygen.
Sulfur and oxygen are in the same column on the periodic table, so they have some similar properties. However, sulfides often have a very strong, sometimes unpleasant, smell. This smell is similar to what you might notice from thiols, which are other sulfur-containing compounds.
Contents
What Are Sulfides Like?
Sulfides have a specific shape where the carbon-sulfur-carbon atoms form an angle. This angle is usually around 99 degrees, which is a bit smaller than the angle you'd find in ethers.
Even though they are similar to ethers, sulfides are generally:
- Less likely to turn into a gas (less volatile).
- Melt at higher temperatures.
- Don't mix as easily with water (less hydrophilic).
These differences happen because the sulfur atom is larger and can be more easily affected by electric fields than an oxygen atom.
Special Sulfides: Thiophenes
There's a unique group of sulfides called thiophenes. These are special because their atoms form a ring that is very stable, a property called aromaticity. Because of this, thiophenes don't act like typical sulfides. For example, they often have a sweet smell instead of a foul one! If you add hydrogen to thiophene, it becomes tetrahydrothiophene, which then acts like a normal sulfide.
Where Do We Find Sulfides?
Sulfides are important in many places, both in nature and in things we make:
- In Living Things: They are found in important building blocks of life, like the amino acid methionine and the vitamin biotin.
- In Petroleum: Crude oil contains many sulfur compounds, including sulfides.
- In Plastics: A strong plastic called polyphenylene sulfide is used for things that need to withstand high temperatures.
- Making Natural Gas: A compound called coenzyme M helps tiny organisms create methane, which is the main part of natural gas.
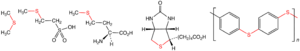
How Are Sulfides Made?
Scientists can make sulfides in different ways in the lab. One common way is by reacting a chemical called a thiol with another compound, often an alkyl halide. This process is called alkylation.
For example, you can combine a thiol (R'SH) with an alkyl bromide (RBr) to get a sulfide (RSR'):
- RBr + HSR' → RSR' + HBr
Another way is to add a thiol to a compound with a double bond, like an alkene. This is called the thiol-ene reaction.
Sulfides can also be made by reacting sulfur dichloride with ethylene, which is how a chemical called mustard gas was produced.
How Do Sulfides React?
Sulfides can undergo several interesting chemical reactions:
Oxidation
Unlike ethers, sulfides can be easily oxidized. This means they can react with oxygen or other oxidizing agents.
- First, a sulfide can be oxidized to a sulfoxide.
- Then, the sulfoxide can be oxidized further to a sulfone.
A common chemical used for this is hydrogen peroxide.
- For example, dimethyl sulfide (S(CH3)2) can be oxidized to dimethyl sulfoxide (OS(CH3)2) and then to dimethyl sulfone (O2S(CH3)2).
Alkylation
Sulfides can easily react with other chemicals to form stable compounds called sulfonium salts. This is different from ethers, which are much harder to alkylate.
- For example, dimethyl sulfide (S(CH3)2) can react with methyl iodide (CH3I) to form trimethylsulfonium iodide ([S(CH3)3]+I-).
This type of reaction is important in living things for moving chemical groups around.
Binding to Metals
Sulfides can attach themselves to certain transition metals, forming what are called thioether complexes. They are considered "soft" ligands, meaning they prefer to bind to "soft" metals.
Breaking Them Down
Sulfides can be broken down by reacting them with hydrogen gas in the presence of certain metals. This process is called hydrogenolysis.
- R−
S−
R' + 2 H
2 → RH + R'H + H
2S
This reaction is used in the petroleum industry to remove sulfur from fuels, making them "sweeter" or less polluting.
Images for kids
See also
 In Spanish: Tioéter para niños
In Spanish: Tioéter para niños


