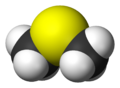
Dimethyl sulfide facts for kids
Dimethyl sulfide (DMS), also known as methylthiomethane, is a special chemical compound. It's the simplest type of thioether, which is a group of compounds that contain sulfur. DMS has a very strong and often unpleasant smell, like cooked cabbage or sulfur. It's a liquid that can easily catch fire and boils at about 37 degrees Celsius (99 degrees Fahrenheit).
You might have smelled DMS before without knowing it! It's part of the smell when you cook certain vegetables like cabbage, corn, and beetroot, and also some seafood. Sometimes, if DMS is present in things like malt (used to make beer), it can mean there are unwanted bacteria growing.
DMS is created naturally when a substance called dimethylsulfoniopropionate (DMSP) breaks down. DMSP is made by tiny living things in the ocean.
Quick facts for kids Dimethyl sulfide |
|
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
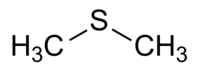
(Methylsulfanyl)methane
|
|
| Other names |
|
| Identifiers | |
| CAS number | |
| PubChem | |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colourless liquid |
| Odor | Stench: cabbage, sulfurous, unpleasant |
| Density | 0.846 g·cm−3 |
| Melting point | |
| Boiling point | |
| Vapor pressure | 53.7 kPa (at 20 °C) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Contents
Where DMS Comes From
Dimethyl sulfide mostly comes from DMSP, which is a very important substance made by tiny marine algae (like plants in the ocean). DMS is the most common sulfur compound released into the Earth's atmosphere by living things. Most of this happens over the oceans, thanks to tiny ocean plants called phytoplankton.
DMS is also made naturally when dimethyl sulfoxide (DMSO) waste is broken down by bacteria in places like sewers. This can sometimes cause bad smells in the environment.
DMS and Earth's Climate
When DMS is released into the air over the ocean, it changes into other sulfur compounds. These include things like sulfur dioxide and sulfuric acid. Sulfuric acid can help create tiny new particles in the air called aerosols. These aerosols can then act like tiny seeds for clouds to form around.
Because DMS helps clouds form, the huge amounts of DMS produced over the oceans might have a big effect on Earth's climate. There's a scientific idea called the CLAW hypothesis. It suggests that DMS might help keep our planet's climate stable, like a natural thermostat.
The Smell of the Sea
Marine phytoplankton also produce dimethyl sulfide. DMS is often called the "smell of the sea." However, it's more accurate to say that DMS is one part of the smell of the sea. Other chemicals, including some that come from DMS, also contribute to this familiar scent.
DMS in Nature's Scents
Dimethyl sulfide, along with similar compounds, has been found in the smells given off by a plant called the dead-horse arum (Helicodiceros muscivorus). This plant smells like rotting meat, which helps it attract insects like flies. These flies then help the plant with pollination.
The Smell of Dimethyl Sulfide
Dimethyl sulfide has a very strong and unique smell, often described as smelling like cabbage. Even a tiny amount of DMS can smell very unpleasant.
DMS is sometimes used as a food additive in very small amounts to give a savory flavor. As mentioned, beetroot, asparagus, cabbage, corn, and seafoods all produce dimethyl sulfide when they are cooked.
DMS is also the main chemical that gives truffles their unique smell. Animals like pigs and special detection dogs are trained to find truffles by sniffing out this compound.
DMS and Life on Other Planets
Scientists are very excited about dimethyl sulfide because it might be a sign of life on other planets! On September 12, 2023, NASA announced that they might have found DMS on an exoplanet called K2-18b. On Earth, DMS is mostly produced by living things. So, finding it on another planet could mean that life might exist there too! This makes DMS a possible "biosignature" – a chemical sign of life.
How DMS Reacts
Dimethyl sulfide can be used in some chemical reactions. For example, it's used to make a compound called borane dimethyl sulfide.
When DMS is exposed to oxygen, it can change into a useful liquid called dimethyl sulfoxide (DMSO). If it's oxidized even more, it can become dimethyl sulfone.
DMS is also used in a process called ozonolysis, which helps break down certain chemical bonds. It's also part of a reaction called the Swern oxidation.
Safety Information
Dimethyl sulfide is very flammable, meaning it can easily catch fire. Its flash point is very low, around -36 degrees Celsius (-33 degrees Fahrenheit). This means it can ignite even at very cold temperatures. Its self-ignition temperature is 205 degrees Celsius (401 degrees Fahrenheit).
DMS can also irritate your eyes and skin. It is harmful if you swallow it. Remember, it has a very unpleasant smell even in tiny amounts.
See also
 In Spanish: Dimetilsulfuro para niños
In Spanish: Dimetilsulfuro para niños
- Coccolithophore, a tiny marine algae that produces DMS
- Dimethylsulfoniopropionate, the parent molecule of DMS in the oceans
- Emiliania huxleyi, a type of coccolithophore that produces DMS
- Phosphine, another molecule that might be a sign of life on other planets
- Swern oxidation, a chemical reaction involving DMS
- Gaia hypothesis, a theory about Earth's living systems
- Geosmin, the substance responsible for the smell of earth
- Petrichor, the earthy scent produced when rain falls on dry soil
Images for kids