Sulfone facts for kids
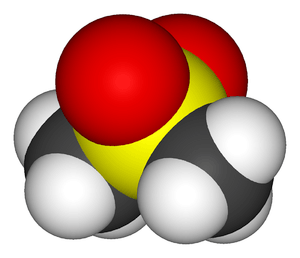
A sulfone is a special type of molecule that has a sulfur atom right at its center. This sulfur atom is connected to other atoms in a very specific way. It forms strong links, called chemical bonds, with two oxygen atoms. These links are extra strong, known as double bonds. The sulfur atom also connects to two other groups of atoms with single bonds.
Sulfones are often created by a process called oxidation. This is like adding oxygen to another type of molecule called a thioether. Think of it as a chemical transformation that builds a sulfone.
Contents
What Are Sulfones?
Sulfones are part of a larger family of chemicals called organosulfur compounds. These are molecules that contain both carbon and sulfur atoms. Sulfones are unique because of how their sulfur atom is bonded to oxygen and other parts of the molecule.
How Sulfones Are Built
Imagine the sulfur atom as the main hub. It has two arms reaching out to grab two oxygen atoms, forming double bonds. These double bonds make the connection very stable. The other two arms of the sulfur atom connect to different groups of atoms. These groups can vary, making each sulfone molecule a bit different.
Making Sulfones in the Lab
Scientists usually make sulfones by taking a simpler molecule, a thioether, and adding oxygen to it. This process is called oxidation. It's a common way to change one type of chemical into another in a laboratory setting.
Uses of Sulfones
Sulfones are not just interesting molecules; they are also very useful! They play important roles in many chemical reactions and are sometimes used in industry.
In Chemical Reactions
One important use of sulfones is in making other chemicals. For example, there's a reaction called the Julia olefination. In this reaction, a sulfone helps to create a new type of molecule called an alkene from an aldehyde. Alkenes are important building blocks for many plastics and other materials.
As Solvents
Sometimes, sulfones are used as solvents. A solvent is a liquid that can dissolve other substances. Think of water dissolving sugar; water is the solvent. Sulfones can be good solvents for certain chemical processes because of their unique structure and properties. They can help mix chemicals that might not normally dissolve well together.
See also
 In Spanish: Sulfona para niños
In Spanish: Sulfona para niños
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |