Brachytherapy facts for kids
Quick facts for kids Brachytherapy |
|
|---|---|
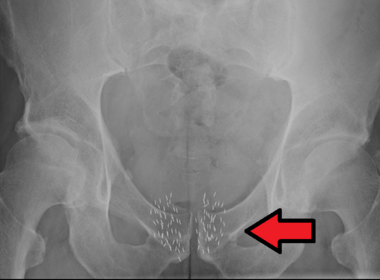
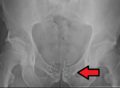
Arrow points to brachytherapy beads used to treat prostate cancer.
|
|
| Other names | internal radiotherapy, sealed source radiotherapy, curietherapy, endocurietherapy |
| Specialty | {{#statements:P1995}} |
| ICD-10-PCS | D?1 |
| ICD-9-CM | 92.27 |
| MeSH | D001918 |
Brachytherapy is a special type of radiation therapy that helps treat cancer. It works by placing tiny, sealed sources of radiation right inside or very close to the area that needs treatment. The word Brachy comes from Greek and means "short."
This treatment is often used for cancers like cervical, prostate, breast, esophageal, and skin cancer. It can also help with tumors in many other parts of the body. Studies show that brachytherapy can be as good as surgery or other radiation treatments, and sometimes even better when used with them. Doctors might use brachytherapy by itself or combine it with surgery, external beam radiotherapy (EBRT), or chemotherapy.
Unlike other treatments where radiation is injected into the body or aimed from outside, brachytherapy uses small, sealed radiation sources placed directly at the cancer. These sources are protected by a capsule or wire. This lets the radiation escape to kill cancer cells nearby, but it stops the radioactive material from moving around or dissolving in body fluids. Sometimes, the capsule is removed later, or it might stay in place, depending on the type of radiation used.
One cool thing about brachytherapy is that the radiation only affects a very small area around the sources. This means healthy tissues farther away get much less radiation. Also, if a patient moves, or if the tumor shifts a little inside the body, the radiation sources stay right where they need to be, next to the tumor. This helps doctors give a very strong dose of radiation directly to the tumor, while keeping healthy parts of the body safe.
Brachytherapy treatments can often be finished faster than other radiation methods. This is good because it gives cancer cells less time to grow between doses. Patients usually need fewer visits to the hospital compared to other treatments, and sometimes they can even go home the same day. This makes the treatment easier and more convenient for many people. Because of these benefits, most patients handle brachytherapy very well.
Contents
- Medical uses of brachytherapy
- Side effects of brachytherapy
- Safety around others
- Types of brachytherapy
- How brachytherapy is done
- History of brachytherapy
- Images for kids
- See also
Medical uses of brachytherapy
Brachytherapy is commonly used to treat cancers of the cervix, prostate, breast, and skin.
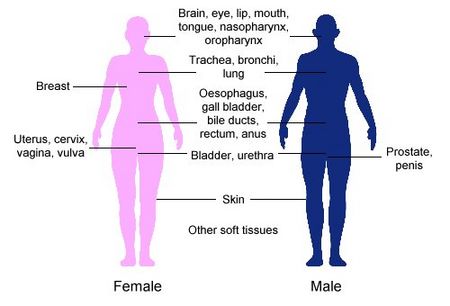
It can also help treat tumors in the brain, eye, head and neck area (like the lip, tongue, and throat), breathing tubes (trachea and bronchi), digestive system (oesophagus, gall bladder, bile-ducts, rectum), urinary system (bladder, urethra), female reproductive system (uterus), and soft tissues.
Because the radiation sources can be placed exactly where the tumor is, brachytherapy can deliver a strong dose of radiation to a small area. The sources stay in place even if the patient or tumor moves, so the radiation remains focused. This helps make sure the whole tumor gets the right amount of radiation. It also lowers the risk of harming healthy tissues or organs nearby, which helps improve the chance of a cure and keeps body functions working well.
High-dose rate (HDR) brachytherapy can also make the total treatment time shorter compared to other radiation methods. Patients often have fewer hospital visits, and the entire radiation plan can be completed more quickly. Many brachytherapy procedures are done without needing to stay overnight in the hospital. This is helpful for people who work, older patients, or those who live far from treatment centers, making it easier for them to get the care they need. Shorter treatment times also make clinics more efficient.
Brachytherapy can be used to cure cancer when tumors are small or have not spread to other parts of the body. For these primary tumors, brachytherapy often works as well as surgery, with similar side effects. However, for larger tumors that are hard to remove with surgery, brachytherapy can offer the only chance of a cure. In more advanced stages of cancer, brachytherapy can also help ease symptoms like pain and bleeding.
If a tumor is hard to reach or too big for brachytherapy alone, doctors might combine it with other treatments like external beam radiation or surgery.
Cervical cancer treatment
Brachytherapy is a common and standard treatment for early or localized cervical cancer in many countries. It can use low-dose rate (LDR), pulsed-dose rate (PDR), or high-dose rate (HDR) methods. When used with external beam radiation, brachytherapy can lead to better results than external beam radiation alone. Its precision allows a strong, targeted dose of radiation to the cervix, while protecting nearby tissues and organs.
The chances of staying cancer-free and living longer are similar for LDR, PDR, and HDR treatments. A big benefit of HDR treatment is that each dose can be given without staying overnight, and it takes a short time, which is very convenient for patients.
Prostate cancer treatment
Brachytherapy for prostate cancer can be done in two ways:
- Permanent LDR seed implantation: Small radioactive "seeds" are placed permanently in the prostate. This is good for patients with a localized tumor and a good outlook. It's very effective at stopping the cancer from coming back. Survival rates are similar to other treatments like surgery, but with fewer side effects such as urinary problems. The procedure is quick, and patients can usually go home the same day and return to normal activities in a day or two. It's often less invasive than surgery to remove the prostate.
- Temporary HDR brachytherapy: This is a newer method, less common than seed implantation. It's mainly used to give an extra boost of radiation after external beam radiation. It delivers a high dose of radiation that matches the tumor's shape in the prostate, while protecting surrounding tissues. Using HDR brachytherapy as a boost can also shorten the overall external beam radiation treatment time.
Breast cancer treatment
Radiation therapy is a standard part of care for women who have had lumpectomy or mastectomy surgery. Brachytherapy can be used after surgery, before chemotherapy, or to relieve symptoms in advanced cases. For breast cancer, HDR temporary brachytherapy is usually performed. After surgery, it can be used as a "boost" following whole breast radiation. More recently, brachytherapy alone is used for "accelerated partial breast irradiation" (APBI), which means radiation is given only to the area right around the original tumor.
The main benefit of breast brachytherapy is that a high dose of radiation can be precisely applied to the tumor, while protecting healthy breast tissues and nearby structures like the ribs and lungs. APBI can usually be completed in about a week. This shorter treatment time, compared to whole breast radiation (which often takes 1-2 months), can be very important for working women, older patients, or those who don't live close to a treatment center.
There are several ways to deliver breast brachytherapy:
Interstitial breast brachytherapy
This involves placing several flexible plastic tubes (catheters) temporarily in the breast tissue. They are carefully positioned to aim radiation at the treatment area while protecting surrounding breast tissue. These tubes connect to a machine called an afterloader, which delivers the planned radiation dose. It can be used as a "boost" after external beam radiation or as APBI.
Intraoperative radiation therapy
Intraoperative radiation therapy (IORT) delivers radiation during the same surgery that removes the tumor. An applicator is placed in the space left after the tumor is removed. A mobile electronic device then creates radiation (either x-rays or electrons) and delivers it through the applicator. The radiation is given all at once, and the applicator is removed before the incision is closed.
Intracavitary breast brachytherapy
Also known as "balloon brachytherapy," this involves placing a single tube into the breast cavity left after tumor removal. A balloon is then inflated through the tube in the cavity. The tube connects to an afterloader, which delivers the radiation dose through the tube and into the balloon. This method is mainly used for APBI.
Some devices combine features of interstitial and intracavitary brachytherapy, using multiple tubes inserted through a single entry point. Studies suggest these devices allow for more precise radiation targeting.
Permanent breast seed implantation
This involves implanting many tiny radioactive "seeds" (like small pellets) into the breast around the tumor site. This is similar to how permanent seeds are used for prostate cancer. The seeds are implanted in a single 1-2 hour procedure and release radiation over several months as they slowly decay. Studies show that the radiation risk from these implants to others (like family members) is safe.
Brain tumor treatment
Surgically Targeted Radiation Therapy (STaRT), also known as GammaTile Therapy, is a type of brachytherapy implant made specifically for use inside the brain. GammaTile is approved to treat newly diagnosed brain tumors and recurrent brain tumors, including meningiomas, metastases, and gliomas. In one study, GammaTile Therapy improved local tumor control without increasing side effects compared to previous treatments for the same site.
Esophageal cancer treatment
For esophageal cancer, brachytherapy is an effective treatment option. It can be used as a "boost" with other radiation therapy or for palliative care to relieve difficulty swallowing. Large applicators or balloon-type tubes are used with an afterloader to expand the esophagus and deliver radiation to the tumor, while protecting nearby healthy tissue. Studies have shown that brachytherapy after external beam radiation or surgery can improve survival rates and reduce the chance of the cancer returning for esophageal cancer patients.
Skin cancer treatment
HDR brachytherapy for non-melanoma skin cancer, like basal cell carcinoma and squamous cell carcinoma, offers an alternative to surgery. This is especially helpful for cancers on the nose, ears, eyelids, or lips, where surgery might cause disfigurement or need a lot of reconstruction. Different applicators can be used to make sure the radiation source is close to the skin, matching its curves for precise delivery of the right radiation dose.
Another type of brachytherapy for skin cancer is Rhenium-SCT (Skin Cancer Therapy). It uses beta rays from Rhenium-188 to treat basal cell or squamous cell carcinomas. The radiation source is in a compound applied on a thin protective foil directly over the lesion. This allows the radiation to be applied to complex areas and minimizes radiation to healthy tissue.
Brachytherapy for skin cancer gives good cosmetic results and works well clinically. Studies up to 5 years show it's very effective at controlling the cancer locally, similar to external beam radiation. Treatment times are usually short, which is convenient for patients. Some experts think brachytherapy might become a standard treatment for skin cancer soon.
Blood vessel treatment
Brachytherapy can be used to treat coronary in-stent restenosis, which is when a blood vessel narrows again after a stent has been placed. A tube is put inside the blood vessel, and radiation sources are inserted and removed through it. While drug-eluting stents are often better for treating in-stent restenosis, there's still interest in brachytherapy for cases where stents have failed or in vein grafts. The therapy has also been looked at for treating narrowing in other blood vessels and even for atrial fibrillation, a heart rhythm problem.
Side effects of brachytherapy
The chances and types of side effects from brachytherapy depend on where the tumor is and what kind of brachytherapy is used. Side effects can be short-term (acute), or long-term.
Short-term side effects
Right after brachytherapy, you might have some bruising, swelling, bleeding, discharge, or discomfort in the treated area. These usually go away within a few days. Patients might also feel tired for a short time after treatment.
For cervical or prostate cancer, brachytherapy can cause temporary urinary problems, like difficulty holding urine or painful urination. You might also have temporary changes in bowel habits, like diarrhea or constipation, or minor rectal bleeding. These usually clear up in a few days or weeks. For permanent prostate cancer seed implants, there's a small chance some seeds might move into the bladder or urethra and pass out in the urine.
For skin cancer, brachytherapy might cause the outer layers of skin to peel in the weeks after treatment, which usually heals in 5–8 weeks. If the cancer is on the lip, an ulcer might form, but it typically heals after 4–6 weeks. Most short-term side effects can be managed with medicine or diet changes and usually disappear once treatment is finished.
Long-term side effects
In a small number of people, brachytherapy can cause long-term side effects if it damages nearby tissues or organs. These are usually mild or moderate. For example, urinary and digestive problems might continue after brachytherapy for cervical or prostate cancer, and might need ongoing care.
For breast or skin cancer, brachytherapy might cause scar tissue to form around the treated area. For breast brachytherapy, fat necrosis (when fatty acids enter breast tissues) can happen, making the breast tissue swollen and tender. This is not harmful and usually occurs 4–12 months after treatment, affecting about 2% of patients.
Safety around others
Many patients wonder if they need to be careful around family and friends after brachytherapy. If temporary brachytherapy is used, no radioactive sources stay in the body after treatment. So, there's no radiation risk to friends or family from being close to you.
If permanent brachytherapy is used, low-dose radioactive seeds are left in the body. The radiation levels are very low and decrease over time. Also, the radiation only affects tissues within a few millimeters of the sources (the tumor being treated). As a precaution, some people with permanent brachytherapy might be advised not to hold small children or be too close to pregnant women for a short time after treatment. Your doctors or nurses can give you specific instructions and tell you how long you need to be careful.
Types of brachytherapy
Different types of brachytherapy are defined by:
- Where the radiation sources are placed in the body.
- How fast or "intense" the radiation dose is.
- How long the radiation dose is delivered.
Source placement
The two main ways to place the radioactive source are interstitial and contact.
- Interstitial brachytherapy: The sources are placed directly into the target tissue, like in the prostate or breast.
- Contact brachytherapy: The radiation source is placed in a space next to the target tissue. This space can be:
- A body cavity (intracavitary brachytherapy), like the cervix or uterus.
- A body tube (intraluminal brachytherapy), like the trachea or oesophagus.
- On the outside of the body (surface brachytherapy), like on the skin.
- In blood vessels (intravascular brachytherapy) for treating problems like coronary in-stent restenosis.
Dose rate
The dose rate of brachytherapy refers to how quickly the radiation is delivered. It's measured in Grays per hour (Gy/h).
- Low-dose rate (LDR) brachytherapy: Radiation sources release radiation slowly, up to 2 Gy per hour. LDR brachytherapy is often used for cancers of the mouth, throat, and prostate cancer.
- Medium-dose rate (MDR) brachytherapy: The dose delivery is medium, between 2 Gy per hour and 12 Gy per hour.
- High-dose rate (HDR) brachytherapy: The dose delivery is fast, more than 12 Gy per hour. HDR brachytherapy is most commonly used for tumors in the cervix, esophagus, lungs, breasts, and prostate. Most HDR treatments are done without needing to stay overnight.
- Pulsed-dose rate (PDR) brachytherapy: This involves short bursts of radiation, usually once an hour, to act like LDR treatment. PDR brachytherapy is often used for gynecological and head and neck cancers.
Duration of dose delivery
Radiation sources can be placed temporarily or permanently.
- Temporary brachytherapy: Radiation sources are placed for a set time (usually minutes or hours) and then removed. The exact time depends on many things, like the dose needed and the type, size, and location of the cancer. In LDR and PDR brachytherapy, the source usually stays in place for up to 24 hours. In HDR brachytherapy, it's typically only a few minutes.
- Permanent brachytherapy: Also called seed implantation, this involves placing small LDR radioactive seeds (about the size of a grain of rice) in the tumor or treatment site and leaving them there permanently. Over weeks or months, the radiation level from the sources slowly drops to almost zero. The inactive seeds then stay in place with no lasting effect. Permanent brachytherapy is most commonly used for prostate cancer.
How brachytherapy is done
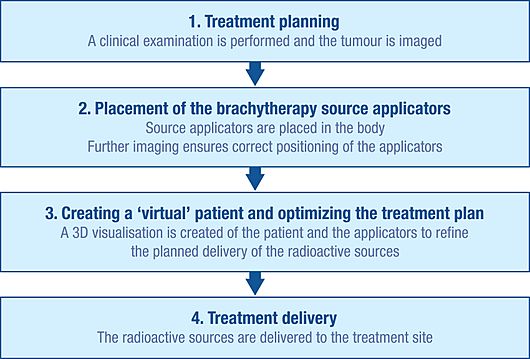
Initial planning
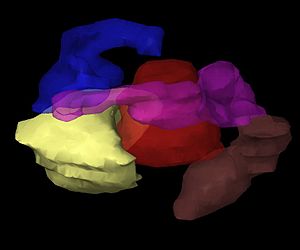
To plan brachytherapy accurately, doctors first do a careful check-up to understand the tumor. They also use imaging tools like x-rays, ultrasound, CT scans, and MRI to see the tumor's shape, size, and how it relates to nearby tissues and organs. This information can be used to create a 3D picture of the tumor and surrounding areas.
With this information, doctors plan the best way to distribute the radiation sources. This includes figuring out how to place the "applicators," which are non-radioactive tools (like needles or plastic tubes) used to deliver the radiation to the treatment site. The type of applicator depends on the cancer and tumor. This careful planning helps avoid areas that get too little radiation (which could mean the treatment doesn't work) or too much radiation (which could cause side effects).
Insertion
Before the radioactive sources can be delivered, the applicators must be inserted and placed correctly according to the plan. Imaging techniques like x-ray, fluoroscopy, and ultrasound are often used to guide the applicators into the right positions and fine-tune the treatment plan. CT scans and MRI can also be used. Once the applicators are in place, they are held securely with stitches or tape to prevent movement. More imaging might be done to help with detailed treatment planning.
Creating a virtual patient
The images of the patient with the applicators in place are put into special treatment planning software. The patient is then moved to a shielded room for treatment. The software turns multiple 2D images of the treatment area into a 3D "virtual patient." In this virtual patient, the exact position of the applicators can be defined. The way the applicators, the treatment area, and the healthy tissues are arranged in the virtual patient is an exact copy of how they are in the real patient.
Optimizing the radiation plan
To find the best way to spread the radiation sources inside the applicators, the treatment planning software lets doctors place virtual radiation sources within the virtual patient. The software shows a picture of how the radiation will spread. This helps the brachytherapy team adjust the source distribution and create a treatment plan that is perfectly suited to each patient's body before the actual radiation begins. This method is sometimes called "dose-painting."
Treatment delivery
The radiation sources used for brachytherapy are always inside a non-radioactive capsule. The sources can be delivered by hand, but more often, a technique called "afterloading" is used. Manual delivery is rare because it risks exposing staff to radiation.
Afterloading involves placing non-radioactive applicators accurately in the treatment site, which are then loaded with the radiation sources. In manual afterloading, a person puts the source into the applicator.
Remote afterloading systems protect healthcare workers from radiation by keeping the radiation source in a shielded safe. Once the applicators are correctly placed in the patient, they are connected to an "afterloader" machine (which holds the radioactive sources) through a series of guide tubes. The treatment plan is sent to the afterloader, which then controls how the sources move along the tubes into the planned positions within the applicator. This process only starts once staff have left the treatment room. The sources stay in place for a specific amount of time, as planned, and then return along the tubes to the afterloader.
After the radioactive sources are delivered, the applicators are carefully removed from the body. Patients usually recover quickly from brachytherapy, which is why it can often be done without an overnight hospital stay.
History of brachytherapy
Brachytherapy started in 1901, soon after Henri Becquerel discovered radioactivity in 1896. Pierre Curie suggested to Henri-Alexandre Danlos that a radioactive source could be put into a tumor. They found that the radiation made the tumor shrink. Separately, Alexander Graham Bell also suggested using radiation this way. In the early 1900s, doctors like Danlos in Paris and Robert Abbe in New York developed techniques for brachytherapy.
An American physicist named William Duane, who worked with the Curies, improved a way to get radon-222 gas from radium solutions. He created radon "seeds" that were used in an early form of brachytherapy. Duane brought this technique back to the United States in 1913 and built Boston's first "radium cow," which produced radon for treating thousands of patients.
In the 1930s, using radium implants was common. Gold seeds filled with radon were used, and later, cobalt needles. Over time, these were replaced by radioactive tantalum and gold, and then iridium became popular. First used in 1958, iridium is now the most common artificial source for brachytherapy.
Interest in brachytherapy decreased in the mid-1900s because of the risk of radiation exposure to the doctors and nurses who manually placed the sources. However, in the 1950s and 1960s, new "remote afterloading systems" were developed. These systems allowed the radiation to be delivered from a shielded safe, which greatly reduced the risk to staff and patients. Along with newer 3D imaging and computer planning systems, these advancements have made brachytherapy a safe and effective cancer treatment today.
The word "brachytherapy" comes from the Greek word brachys, meaning "short-distance" or "short."
Images for kids
See also
 In Spanish: Braquiterapia para niños
In Spanish: Braquiterapia para niños