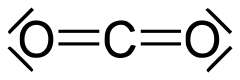Carbonyl group facts for kids
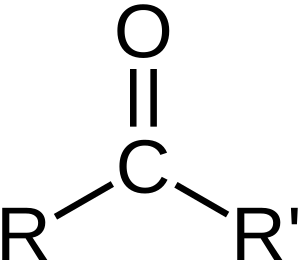
In organic chemistry, a carbonyl group is a special group of atoms. It has a carbon atom connected to an oxygen atom with a double bond. This means they share two pairs of electrons. You can find this group in many types of organic chemicals. These include aldehydes, ketones, and carboxylic acids. A chemical that contains a carbonyl group is often called a carbonyl compound.
The word "carbonyl" can also mean carbon monoxide when it acts as a ligand. This happens in inorganic or organometallic compounds. An example is nickel carbonyl. But this article is about the carbonyl group in organic chemistry.
Contents
What are Carbonyl Compounds?
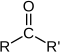
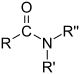
In organic chemistry, the carbonyl group is a key part of many different compounds. Here are some common types:
| Compound | Aldehyde | Ketone | Carboxylic acid | Carboxylate ester | Amide |
| Structure | 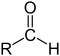 |
 |
 |
||
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | RCONR'R'' |
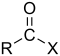
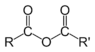
| Compound | Enone | Acyl halide | Acid anhydride | Imide |
| Structure | 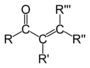 |
 |
 |
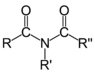 |
| General formula | RC(O)C(R')CR''R''' | RCOX | (RCO)2O | RC(O)N(R')C(O)R'' |
In these formulas, 'R' usually stands for a chain of carbon and hydrogen atoms. It can be different in each compound.
Other organic compounds with carbonyl groups include urea and carbamates. Also, there are chloroformates and phosgene, which come from acyl chlorides. You can also find them in carbonate esters, thioesters, lactones, and lactams. Hydroxamates and isocyanates also have them.
Some inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide.
How Carbonyl Groups Work
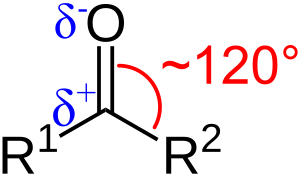
The carbon-oxygen double bond (C=O) in organic compounds is usually about 120 picometers long. This is a very tiny distance. In inorganic carbonyls, this bond can be a bit shorter. For example, in carbon monoxide, it's 113 picometers.
The carbon atom in a carbonyl group is often "electrophilic". This means it likes to attract electrons. Think of it like a magnet for electrons. Aldehydes are more electrophilic than ketones. Ketones are more electrophilic than esters. And esters are more electrophilic than amides.
Because the carbon atom is slightly positive and the oxygen atom is slightly negative, the C=O bond is "polar". This polarity makes the carbonyl group very reactive. Many other chemicals, called "nucleophiles", like to attack the carbon atom. When this happens, the carbon-oxygen double bond can break. This leads to new chemical reactions.
The C=O bond also makes any hydrogen atoms on the carbon next to it more acidic. This means those hydrogen atoms can be easily removed in a chemical reaction.
How We Study Carbonyl Groups
Scientists use special tools to study carbonyl groups. These tools help them figure out if a chemical has a carbonyl group and how it's structured.
- Infrared Spectroscopy: This method uses infrared light. The C=O double bond absorbs infrared light at specific "wavenumbers". These are usually between 1600 and 1900 cm−1. This absorption is like a unique "fingerprint" for the carbonyl group. It helps scientists identify it.
- Nuclear Magnetic Resonance (NMR): This method looks at the nuclei of atoms. The carbon atom in a carbonyl group shows up in a specific range in a 13C NMR spectrum. This range is usually between 160 and 220 parts per million (ppm). This also helps scientists confirm the presence of a carbonyl group.
See also
- Carbon–oxygen bond
- Organic chemistry
- Functional group
- Bridging carbonyl
- Electrophilic addition
 In Spanish: Grupo carbonilo para niños
In Spanish: Grupo carbonilo para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |