Cracking (chemistry) facts for kids
Cracking is a special process in chemistry where big, complicated molecules are broken down into smaller, simpler ones. Think of it like taking a long LEGO chain and breaking it into smaller, more useful pieces! This happens by breaking the bonds between carbon atoms inside the molecules.
This process is also sometimes called pyrolysis. It's really important in the petroleum industry.
Contents
What is Cracking?
Cracking is all about changing large hydrocarbon molecules into smaller ones. Hydrocarbons are special compounds made only of carbon and hydrogen atoms. They are found in things like oil and natural gas.
Imagine you have a very long chain of carbon and hydrogen atoms. Cracking breaks this long chain into shorter ones. These shorter chains are much more useful for us.
Why is Cracking Important?
Cracking is super important because it helps us make valuable products from petroleum (also known as crude oil). Crude oil contains many large, heavy hydrocarbons that aren't very useful on their own.
By cracking these big molecules, we can create "lighter" products. These include:
Without cracking, we wouldn't have enough gasoline or other important fuels from crude oil.
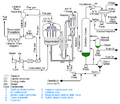
How Does Cracking Work?
The cracking process usually needs a lot of temperature (heat). Sometimes, special substances called catalysts are also used. Catalysts are like helpers that speed up the chemical reaction without being used up themselves.
When big molecules, like a type of hydrocarbon called an alkane, are heated or mixed with a catalyst, their carbon-carbon bonds break. This turns them into smaller alkanes and another type of hydrocarbon called an alkene. Alkenes are special because they have a double bond between two carbon atoms.
Images for kids
-
A refinery using the Shukhov cracking process in Baku, Soviet Union, 1934.
See also
 In Spanish: Craqueo para niños
In Spanish: Craqueo para niños