Ellagic acid facts for kids
Quick facts for kids Ellagic acid |
|
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
2,3,7,8-Tetrahydroxy[1]benzopyrano[5,4,3-cde][1]benzopyran-5,10-dione
|
|
| Other names | 4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone |
| Identifiers | |
| CAS number | |
| PubChem | |
| DrugBank | DB08468 |
| KEGG | C10788 |
| ChEBI | CHEBI:4775 |
| SMILES | O=C1Oc3c2c4c1cc(O)c(O)c4OC(=O)c2cc(O)c3O |
|
InChI
InChI=1/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Density | 1.67 g/cm3 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
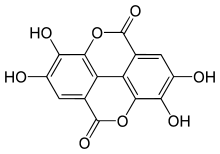
Ellagic acid is a special natural chemical found in many fruits and vegetables. It belongs to a group of compounds called polyphenols. These are chemicals that plants make, and they often have good effects on our bodies. Ellagic acid is also known as a dilactone, which means it has two special ring-like structures in its chemical makeup.
Contents
What's in a Name?
The name "ellagic acid" comes from a French word, acide ellagique. It's a bit of a clever name because it's the word galle (which means galls, like the bumps on oak trees) spelled backward. This is because ellagic acid can be found in these galls. The name helps tell it apart from another similar chemical called gallic acid. If you look at the chemical structure, ellagic acid looks like two gallic acid molecules joined together.
How Ellagic Acid Works
How Plants Make It
Plants create ellagic acid from other compounds called tannins. These tannins are complex chemicals found in many plants, like ellagitannin and geraniin. When these tannins break down, they release ellagic acid.
How Our Bodies Use It
When we eat foods with ellagic acid, our bodies don't absorb all of it directly. Instead, tiny helpers in our gut, called gut flora (which are good bacteria), break it down. They turn ellagic acid into other compounds called Urolithins. These urolithins are easier for our bodies to use. About 90% of the ellagic acid we eat gets changed by these gut bacteria before our bodies can absorb it.
A Look Back in Time
Ellagic acid was first discovered by a chemist named Henri Braconnot in 1831. Later, in 1905, Maximilian Nierenstein found ellagic acid in many different plants. These included algarobilla, dividivi, oak bark, pomegranate, myrabolams, and valonea. He also thought that a type of mold called Penicillium might help create it. The first person to make ellagic acid in a lab was Julius Löwe. He did this by heating gallic acid with arsenic acid or silver oxide.
Where to Find Ellagic Acid
Ellagic acid is naturally present in many plants and foods. You can find it in different types of oak trees, like the North American white oak (Quercus alba) and the European red oak (Quercus robur). A water plant called Myriophyllum spicatum also produces ellagic acid. Even some medicinal mushrooms, like Phellinus linteus, contain it.
In Your Food
Some of the foods with the most ellagic acid are raw chestnuts, walnuts, pecans, cranberries, raspberries, strawberries, and grapes. You can also find it in peaches and pomegranates. It's even in some distilled beverages!
Here's a table showing how much ellagic acid is in some common foods:
| Dietary source | Ellagic acid |
|---|---|
| Fruits (mg/100g fresh weight) | |
| Blackberries | 150 |
| Black raspberries | 90 |
| Boysenberries | 70 |
| Cloudberries | 315.1 |
| Pomegranate | 269.9 |
| Raspberries | 270 |
| Rose hip | 109.6 |
| Strawberries | 77.6 |
| Strawberry jam | 24.5 |
| Yellow raspberries | 1900 |
| Nuts (mg/g) | |
| Pecans | 33 |
| Walnuts | 59 |
| Beverages (mg/L) | |
| Pomegranate juice | 811.1 |
| Cognac | 31–55 |
| Oak-aged red wine | 33 |
| Whiskey | 1.2 |
| Seeds (mg/g) | |
| Black raspberries | 6.7 |
| Red raspberries | 8.7 |
| Boysenberries | 30 |
| Mango | 1.2 |
Research and Health Claims
Ellagic acid has been sold as a dietary supplement with claims that it can help with various health issues, including serious diseases. However, it's important to know that there is currently no strong scientific evidence to support these claims. Health organizations advise people to be careful about products that make big promises without scientific proof. Always rely on information from trusted doctors and scientists when it comes to your health.
See also
 In Spanish: Ácido elágico para niños
In Spanish: Ácido elágico para niños