Lead-acid battery facts for kids

12-volt lead-acid car battery
|
|
| Specific energy | 35–40 Wh/kg |
|---|---|
| Energy density | 80–90 Wh/L |
| Specific power | 180 W/kg |
| Charge/discharge efficiency | 50–95% |
| Energy/consumer-price | 7 (sld) to 18 (fld) Wh/US$ |
| Self-discharge rate | 3%–20%/month |
| Cycle durability | <350 cycles |
| Nominal cell voltage | 2.1 V |
| Charge temperature interval | Min. −35°C, max. 45°C |
A lead-acid battery is a special type of rechargeable battery. It was first invented in 1859 by a French scientist named Gaston Planté. This was the very first rechargeable battery ever made!
Compared to newer batteries, lead-acid batteries don't store as much energy for their size. But they are great at giving a lot of power very quickly. This makes them perfect for starting car engines. They are also not very expensive.
Even today, lead-acid batteries are used a lot because they are cheap. In 1999, they made up almost half of all battery sales worldwide. You can find large lead-acid batteries used as backup power. They power things like cell phone towers and hospitals. They also work in places that don't have a regular power grid. Some types, like gel-cells and absorbed glass-mat (AGM) batteries, are called VRLA (valve-regulated lead-acid) batteries. These are sealed and need less care.
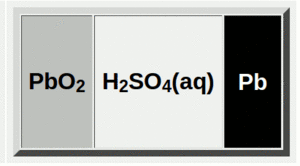
When a lead-acid battery is fully charged, it stores energy. This energy is held between lead metal on one side and lead dioxide on the other.
Contents
- History of Lead-Acid Batteries
- How Lead-Acid Batteries Work
- Checking the Battery's Charge
- Battery Construction
- What Lead-Acid Batteries Are Used For
- Battery Lifespan and Use
- Sulfation: A Battery Problem
- Stratification: Acid Separation
- Risk of Explosion
- Environmental Concerns
- Battery Additives
- Corrosion Problems
- Images for kids
- See also
History of Lead-Acid Batteries
In 1801, a French scientist named Nicolas Gautherot noticed something interesting. Wires used for experiments could create a small electric current on their own. This happened even after the main power was turned off.
Then, in 1859, Gaston Planté created the first battery that could be recharged. He made his first battery from two sheets of lead. He separated them with rubber strips and rolled them into a spiral. These early batteries were used to light up train cars when they stopped at stations.
In 1881, Camille Alphonse Faure made an even better design. He used a lead grid that was filled with a special lead oxide paste. This new design was much easier to make in large numbers. An early company that made these batteries was started by Henri Tudor in 1886.
Later, people found ways to make batteries that wouldn't leak. They used a gel instead of a liquid inside. These gel batteries could be used in different positions. In the 1970s, the VRLA battery was invented. This included modern AGM types. These batteries are sealed and can work in almost any position.
How Lead-Acid Batteries Work
Discharging the Battery
When a lead-acid battery is used, it's called "discharging." During discharge, both the positive and negative plates change. They become a material called lead(II) sulfate. The liquid inside, called the electrolyte, also changes. It loses most of its sulfuric acid and becomes mostly water.
This chemical process creates electricity. Electrons move from the negative plate, through your device, and to the positive plate. This flow of electrons is what powers things.
Charging the Battery
When you charge the battery, the opposite happens. The lead sulfate on the plates changes back. The negative plate becomes lead again. The positive plate turns back into lead dioxide. The liquid inside gets more sulfuric acid. This is how the battery stores energy again.
If you charge the battery too much, it can create oxygen and hydrogen gas. These gases can bubble out of the battery. Some lead-acid batteries let you add pure water to replace what's lost.
Freezing in Cold Weather
The liquid inside the battery can freeze in very cold weather. This is more likely to happen when the battery is low on charge. When the battery is low, the liquid has less sulfuric acid and more water. Water freezes more easily than sulfuric acid.
How Ions Move
Inside the battery, tiny charged particles called ions move around. When the battery is discharging, hydrogen ions move to the positive plate. Other ions move to both plates. This movement helps the chemical reactions happen. When you charge the battery, the ions move in the opposite direction.
The liquid inside the battery can also move around. This helps the battery charge and discharge more efficiently.
Checking the Battery's Charge
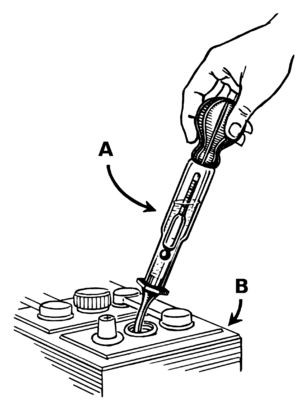
One cool thing about lead-acid batteries is how easy it is to check their charge. You can measure the "specific gravity" of the liquid inside. This is how dense the liquid is. As the battery discharges, the liquid becomes less dense.
Some batteries even have a simple tool called a hydrometer. It has little colored balls that float. How many float tells you the charge level. In old submarines, sailors would check this often. It told them how long the submarine could stay underwater.
You can also check the battery's voltage. A fully charged 12-volt car battery usually shows around 12.6 volts. If it's much lower, it needs charging.
Battery Construction
Plates Inside the Battery
The main parts of a lead-acid battery are its plates. These are where the chemical reactions happen. Early batteries used simple lead sheets. But these didn't produce much power.
Gaston Planté found a way to make the plates have a much larger surface area. He rolled lead foil into spirals. This made the battery more powerful.
Later, Camille Alphonse Faure invented a better way. He coated a lead grid with a special paste made of lead oxides. This made batteries much easier and cheaper to build. This method is still used today!
Modern plates are designed to be stronger and carry more electricity. They often have fiberglass mats to help hold the paste. The lead used for the grids is also mixed with other metals. For example, lead-calcium or lead-selenium alloys are used. These make the battery last longer and need less maintenance.
Modern Battery Design
Today's battery paste has special ingredients. These include carbon black and barium sulfate. These help the battery work better and last longer. They also help the plates change back to their original state when charging.
Once the plates are dry, they are stacked up. Thin sheets called "separators" are placed between them. These separators stop the positive and negative plates from touching. If they touched, it would cause a short circuit. Separators allow ions to flow through them.
In multi-cell batteries, the cells are connected in a series. This means the positive of one cell connects to the negative of the next. This increases the total voltage. All connections are made of lead alloy to prevent corrosion.
Some batteries, called "deep-cycle batteries," have different positive plates. Instead of flat plates, they use lead-oxide cylinders or tubes. This design gives them more surface area. It allows them to give out more power quickly. This is good for things like forklifts or starting large marine engines.
About 60% of a car battery's weight comes from lead parts. The rest is the liquid, separators, and the case.
Separators
Separators are very important. They are thin sheets placed between the positive and negative plates. Their job is to prevent the plates from touching and causing a short circuit. They also let ions move between the plates.
Separators used to be made of wood or ceramic. Now, they are often made of plastic. A good separator must be strong and let ions pass through easily. It must also resist the acid inside the battery.
Absorbent Glass Mat (AGM) Batteries
In AGM batteries, the separators are made of a special glass fibre mat. This mat is soaked in the battery's liquid. There's just enough liquid to keep the mat wet. If the battery case breaks, the liquid won't spill out.
A big advantage of AGM batteries is that gases can move through the glass mat easily. If hydrogen or oxygen gas is made during charging, it can pass through the mat. This helps the gases turn back into water. In older batteries, these gases would escape into the air.
Because the gases recombine, AGM batteries can be completely sealed. This makes them good for portable devices. They can also be installed in any direction. AGM batteries also have a special one-way valve. This valve lets gas out if the pressure inside gets too high. This is why they are called "valve-regulated."
Another benefit is that the glass mat makes the plates very strong. This allows the plates to be packed tightly. This means AGM batteries can store more energy for their size.
AGM batteries also help prevent "stratification." In normal batteries, the heavier acid can sink to the bottom. This makes the top part of the battery weaker. The glass mat in AGM batteries stops this from happening. This helps the battery last longer and work better in cold weather.
Gelled Electrolytes (Gel Batteries)
In the 1970s, another sealed battery type was developed: the gel battery. Instead of liquid, it uses a special silica gel mixed into the electrolyte. This turns the liquid inside into a thick, jelly-like paste.
Gel batteries have many of the same benefits as AGM batteries. They don't evaporate easily and need very little maintenance. They also work well in extreme hot or cold conditions.
However, gel batteries have one small drawback. The gel makes it harder for ions to move quickly. This means they can't deliver a huge burst of power as fast as other types. So, gel batteries are often used for storing energy. They are great for systems that are not connected to the main power grid.
"Maintenance-Free" and "Sealed" Batteries
Both gel and AGM batteries are sealed. They don't need water added, can be used in any position, and have a valve to release gas. Because of this, they are often called "maintenance-free" or "sealed" batteries. They are also known as VRLA (valve-regulated lead-acid) batteries.
While they need less care, they still need some attention. Over time, they can "dry out" and lose power. Regular checks can help you know if they need more care.
VRLA batteries became popular for motorcycles around 1983. The acid is absorbed, so it won't spill. They also handle vibrations better. They are also used in places like phone towers because they are compact and flexible.
What Lead-Acid Batteries Are Used For
Most lead-acid batteries are used in cars. They are called SLI batteries, which stands for Starting, Lighting, and Ignition. About 320 million of these were shipped in 1999.
Large, stationary lead-acid batteries are used for backup power. You can find them in telephone and computer centers. They also store energy for power grids and homes not connected to the grid. They are used for emergency lighting and to power sump pumps during power outages.
Traction batteries are used in electric vehicles. This includes golf carts and other electric cars. Big lead-acid batteries also power electric motors in submarines when they are underwater. They are also emergency power for nuclear submarines.
Sealed VRLA batteries are used in many portable devices. These include uninterruptible power supplies (UPS) for computers. They also power electric scooters, wheelchairs, and electrified bicycles. Many electric forklifts use them too. The battery's weight helps balance the forklift.
Battery Lifespan and Use
Starting Batteries
Car batteries are made for starting engines. They have many thin plates to give a lot of power quickly. They are not made to be deeply discharged. If you drain them too much, they can get damaged and fail early.
Starting batteries should not be left fully discharged. They should be charged regularly to prevent damage. They are also lighter than deep-cycle batteries. This is because their plates don't go all the way to the bottom. This allows loose material to fall off without causing problems.
Deep-Cycle Batteries
Deep-cycle batteries are designed differently. They are made to be discharged and recharged many times. They are used in things like photovoltaic (solar) systems and electric vehicles.
These batteries have thicker plates. They can't give as much sudden power as car batteries. But they can handle being discharged often without getting damaged.
Some batteries are a mix of both types. They are called "marine/motorhome" or "leisure batteries." They can be discharged more than car batteries but less than true deep-cycle ones.
Fast and Slow Charging
How much power a lead-acid battery can hold depends on how fast you use it. This is explained by something called Peukert's law.
When you charge or discharge a battery, the changes first happen on the surface of the plates. Over time, these changes spread throughout the whole plate.
If a battery is fully drained and you charge it very quickly for a short time, only the surface of the plates gets charged. The battery voltage might look high, but it's not fully charged inside. After a few hours, this surface charge spreads out. Then, the battery might not have enough power to start a car. As long as you don't overcharge it too much, the battery usually won't be damaged. It should eventually return to a normal charged state.
Sulfation: A Battery Problem
Lead-acid batteries can lose their ability to charge if they stay discharged for too long. This problem is called sulfation. It happens when lead sulfate turns into hard crystals on the plates.
When a battery works, lead and lead dioxide react with sulfuric acid to form lead sulfate. Normally, this lead sulfate easily changes back when you recharge the battery. But if the battery is left discharged, some lead sulfate doesn't change back. It turns into a stable, hard crystal form. These crystals don't dissolve when you recharge.
This means less active material is available to create electricity. Sulfation makes it harder to recharge the battery. The sulfate deposits can even crack the plates. This greatly reduces the battery's power and lifespan. You might see a white coating on the plates if you look inside.
Batteries with sulfation have high internal resistance. They can only give a small amount of power. They also take longer to charge and get hotter.
You can avoid sulfation by always fully recharging the battery right after you use it. There are no proven ways to reverse severe sulfation. So, preventing it is the best approach.
Stratification: Acid Separation
A normal lead-acid battery has a mix of water and acid. Sulfuric acid is heavier than water. So, when the battery charges, the acid can sink to the bottom. This is called "stratification."
Over time, the acid will mix evenly again, but it's a very slow process. If you often charge and discharge the battery only partly, stratification gets worse. This reduces the battery's power. It also causes corrosion at the top of the plates and sulfation at the bottom.
To fix stratification, you can overcharge the battery a little bit. This creates gas bubbles that mix the liquid. Batteries in moving vehicles also get mixed up from bumps and turns.
Risk of Explosion
Charging a battery too much can cause a process called "gassing." This creates hydrogen and oxygen gas. Wet cell batteries have open vents to let this gas out. VRLA batteries have special valves for this.
Sometimes, these gases can build up and ignite. This can cause an internal explosion. The explosion can burst the battery case. It can spray acid and pieces of the battery. An explosion in one cell can even set off gas in other cells.
If you are in a poorly ventilated area, sparks can also cause an explosion. This can happen when you connect or disconnect wires from the battery.
If VRLA batteries swell up, it's a sign that pressure is building inside. These batteries should be handled carefully and thrown away safely. Anyone working near batteries should protect their eyes and skin. Wear a face shield, overalls, and gloves to stay safe.
Environmental Concerns
Lead-acid batteries contain lead, which can be very toxic. Long-term exposure to lead can harm the brain, kidneys, and hearing. It can also cause learning problems in children. The car industry uses a lot of lead every year, mostly for batteries.
Even though lead recycling is common, some lead still ends up in landfills. Also, lead can be released during mining and manufacturing. People are trying to find other types of batteries because of these environmental worries. But lead-acid batteries are still popular because they are cheap.
Recycling Batteries
Recycling lead-acid batteries is one of the most successful recycling programs. In the United States, almost all battery lead was recycled between 2014 and 2018.
However, recycling lead can still cause pollution. It can also harm people's health. Some reports say that lead-acid battery recycling is one of the most dangerous industrial processes for human health.
Recycling sites themselves can become sources of lead pollution. It's very important for recycling plants to have good pollution control systems. They need to keep improving to meet safety standards.
Battery Additives
For a long time, people have added chemicals to lead-acid batteries. They hoped these additives would reduce lead sulfate buildup and improve battery life. But these treatments are rarely helpful.
Two common additives are Epsom salts and EDTA. Epsom salts might slightly help a weak battery for a short time. EDTA can dissolve sulfate deposits. But once dissolved, this material can't be used by the battery anymore. So, a battery "revived" with EDTA will have a shorter life.
Additives cannot fix physical problems inside the battery. They can't fix cracked plates, corroded connectors, or damaged separators.
Corrosion Problems
You might see corrosion on the outside metal parts of a lead-acid battery. This happens when the battery terminals and connectors react with chemicals.
White corrosion is usually lead or zinc sulfate crystals. If you have aluminum connectors, they might corrode into aluminum sulfate. Copper connectors can turn blue and white. You can reduce corrosion by putting petroleum jelly on the terminals.
If you overfill the battery with water, the liquid can expand when hot. It might leak out of the vents onto the top of the battery. This liquid can then react with the metal parts and cause corrosion.
Acid fumes can also escape through the vents, especially if the battery is overcharged. These fumes can build up and react with any exposed metal.
Images for kids
-
Car lead-acid battery after explosion showing brittle fracture in casing ends
- Automotive battery
- Battery room
- Comparison of commercial battery types
- Dry cell
- History of the battery
- List of battery sizes
- List of battery types
- Silver–calcium battery
- UltraBattery
- Peukert's law
- Lithium-ion battery
- Vanadium redox battery
- Sodium-ion battery
- Sodium–sulfur battery
See also
 In Spanish: Batería de plomo y ácido para niños
In Spanish: Batería de plomo y ácido para niños
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |