Nickel–cadmium battery facts for kids

From top to bottom: "Gumstick", AA, and AAA Ni-Cd batteries
|
|
| Specific energy | 40–60 W·h/kg |
|---|---|
| Energy density | 50–150 W·h/L |
| Specific power | 150 W/kg |
| Charge/discharge efficiency | 70–90% |
| Self-discharge rate | 10%/month |
| Cycle durability | 2,000 cycles |
| Nominal cell voltage | 1.2 V |
The nickel-cadmium battery (often called Ni-Cd battery or NiCad battery) is a special kind of rechargeable battery. It uses two main parts called electrodes: one made of nickel oxide hydroxide and the other of metallic cadmium. The short name Ni-Cd comes from the chemical symbols for nickel (Ni) and cadmium (Cd). The name NiCad is actually a brand name, but many people use it for all Ni-Cd batteries.
These batteries were first invented in 1899. A Ni-Cd battery usually gives about 1.2 volts of power. This voltage stays pretty steady until the battery is almost empty. Ni-Cd batteries come in many sizes, from small ones that fit in portable devices to large ones used for backup power. They are known for lasting a long time and working well in cold temperatures. A big advantage is that they can give out a lot of power very quickly. However, the materials used are more expensive than those in lead-acid batteries. Also, they lose their charge faster when not in use.
In the past, sealed Ni-Cd batteries were popular in power tools, cameras, flashlights, and remote-controlled toys. But now, nickel-metal hydride batteries and lithium-ion batteries are more common. This is because they can store more power and are cheaper. Also, cadmium is a toxic metal, so getting rid of old Ni-Cd batteries can be bad for the environment. Because of this, in places like the European Union, Ni-Cd batteries are mostly used for special equipment like medical devices.
Larger Ni-Cd batteries, called "vented wet cell" batteries, are still used in emergency lighting, backup power systems, and other important jobs.
Contents
History of Ni-Cd Batteries
The very first Ni-Cd battery was made by Waldemar Jungner in Sweden in 1899. Back then, the main competitor was the lead-acid battery, which wasn't as strong or long-lasting. Jungner's new battery quickly became much better at storing energy.
In 1902, Thomas Edison also worked on similar batteries. By 1906, Jungner had a factory making these batteries.
Later, in 1932, new ways were found to put the active materials inside the battery. By 1947, scientists started working on sealed Ni-Cd batteries, which are the kind we know today.
Production of these batteries in the United States began in 1946. At first, they were "pocket type" batteries. Around the mid-1900s, "sintered-plate" Ni-Cd batteries became popular. These plates are made by pressing nickel powder together, making them very porous. This design allows for more power and lower internal resistance.
Since the 2000s, most Ni-Cd batteries for everyday use are made with a "jelly-roll" design. This means the parts inside are rolled up tightly like a jelly roll.
How Ni-Cd Batteries Work
Ni-Cd batteries have a special design that lets them give out a lot of power. For example, a common AA-size Ni-Cd cell can give about 1.8 amperes of power. Larger D size batteries can give up to 3.5 amperes.
Some special Ni-Cd batteries, used in things like model airplanes, can provide huge currents, sometimes up to 100 amps! This allows models to fly for several minutes with good power. However, newer batteries like lithium polymer (Lipo) and lithium iron phosphate (LiFe) batteries are now even better for these uses.
Battery Voltage
A Ni-Cd cell usually has a voltage of 1.2 volts (V). This is a bit lower than the 1.5 V of regular alkaline batteries. Because of this, Ni-Cd batteries might not work in every device that uses alkaline batteries.
However, the 1.5 V of an alkaline battery is its starting voltage, which drops quickly as it's used. A Ni-Cd battery's voltage stays very close to 1.2 V until it's almost completely empty. Many electronic devices are made to work even when a battery's voltage drops to 0.9 V or 1.0 V. So, the steady 1.2 V from a Ni-Cd battery is usually enough. One small problem is that because the voltage stays so steady, it can be hard to tell when the battery is running low.
Ni-Cd batteries that replace 9 V batteries usually have six cells, giving 7.2 volts. Some special ones have seven cells for 8.4 volts.
Charging Ni-Cd Batteries
You can charge Ni-Cd batteries at different speeds. The charging speed depends on how much current you feed into the battery compared to its capacity. You always need to put more energy into the battery than its actual capacity because some energy is lost as heat during charging.
For a slow "overnight" charge, you might charge a 100 mAh battery with 10 mA for 14-16 hours. This means you put in a total of 140-160 mAh. For a faster charge, you might charge at 100% of the battery's capacity in 1 hour (called 1C). A 100 mAh battery would take about 1 hour and 15 minutes to charge fully at this rate. Some very special batteries can charge in just 10-15 minutes, but this is rare. Faster charging can make the battery get very hot and might even damage it.
Ni-Cd batteries work best between -20 °C and 45 °C. When charging, the battery usually stays cool at first. But as it gets full, its temperature can rise to 45-50 °C. Some smart chargers can sense this temperature rise and stop charging to prevent damage.
When not in use, a Ni-Cd battery will slowly lose its charge. This is called "self-discharge." Older Ni-Cd batteries might lose about 10% of their charge per month. Newer ones are much better, losing only 1% or 2% per month. You can use a very small "trickle charge" to keep a battery fully charged. If you're storing a battery for a long time, it's best to discharge it to about 40% capacity or even fully discharge it, and then store it in a cool, dry place.
Overcharging Issues
Sealed Ni-Cd batteries are designed to hold gases that form inside during charging. These gases usually turn back into water. But if you overcharge the battery too much, especially during fast charging, the pressure inside can get too high. If the pressure relief valve opens, some water in gas form can escape. Losing this water can quickly reduce how much power the battery can hold and deliver. Cheap chargers might not be smart enough to prevent overcharging, which can damage even good quality batteries.
Inside a Ni-Cd Battery
A fully charged Ni-Cd battery contains:
- A positive plate made of nickel(III) oxide-hydroxide.
- A negative plate made of cadmium.
- A separator to keep the plates apart.
- An alkaline liquid called an electrolyte, usually potassium hydroxide.
Ni-Cd batteries usually have a metal case with a special seal and a safety valve. The positive and negative plates are rolled up in a spiral shape inside the case, separated by the separator. This "jelly-roll" design allows the battery to deliver a much higher current than a regular alkaline battery of the same size.
The chemical reactions inside the battery allow it to store and release electricity. When the battery is used (discharging), the cadmium and nickel oxide hydroxide change. When it's recharged, they change back. The electrolyte liquid is not used up in these reactions.
When Jungner first made Ni-Cd batteries, he used slightly different materials. It took a long time for scientists to fully understand the exact chemical reactions happening inside.
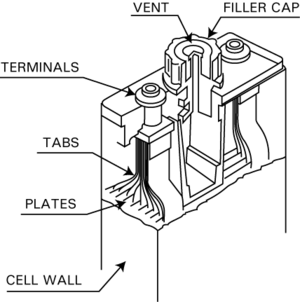
Vented Cell Batteries
Vented cell Ni-Cd batteries are also called wet cell or flooded cell batteries. They are used when you need a lot of power or very high discharge rates. Unlike the sealed Ni-Cd batteries, vented cells have a small vent or valve. This valve lets out any gases (oxygen and hydrogen) that build up if the battery is overcharged or discharged very quickly.
Because they can release gas, these batteries are safer and lighter. They are also not easily damaged by too much charging or discharging. You'll find them in airplanes, trains, backup power systems for phones, and for starting large engines. Vented cell Ni-Cd batteries can last a very long time, sometimes 20 years or more, and work well in extreme temperatures (from -40 to 70 °C).
These batteries are made of cells connected together in a steel box to get the right voltage (1.2 V per cell). Each cell is usually made of strong plastic, with many nickel-cadmium plates welded inside. A rubber separator keeps the plates apart. The cells are filled with a liquid electrolyte, which is a solution of potassium hydroxide. The liquid level needs to be checked and refilled sometimes because water can evaporate or be released as gas.
Charging vented cells is usually done in stages. They are charged at a steady current until they reach a certain voltage. Then, they get a "top-up" charge to make sure all gases are removed from the electrodes and the electrolyte level is correct.
Uses of Ni-Cd Batteries
Small, sealed Ni-Cd batteries can be used alone or put together in battery packs. They are used in portable electronics and toys, often in the same sizes as regular batteries (like AAA, AA, C, D). When you use Ni-Cd batteries instead of regular ones, remember they have a slightly lower voltage and might not last as long in some devices. Small button-shaped Ni-Cd cells are used in cameras, small lamps, and computer memory backup.
Special Ni-Cd batteries are used in cordless phones, emergency lights, and other devices. They can provide a lot of power quickly because they have low internal resistance. This makes them great for remote-controlled model planes, boats, and cars, as well as cordless power tools and camera flashes.
Larger vented cells are used for starting aircraft engines, in electric vehicles, and for backup power systems.
Popularity and Availability
Over the years, making batteries has become much cheaper, and more devices use batteries. Around the year 2000, about 1.5 billion Ni-Cd batteries were made each year. Until the mid-1990s, Ni-Cd batteries were the most popular rechargeable batteries for home electronics.
However, because of environmental concerns about cadmium, the European Union passed a rule in 2006. This rule limited the sale of Ni-Cd batteries to consumers for portable devices. They are now mostly allowed only for medical devices, alarm systems, emergency lighting, and some power tools. The use in power tools was also banned in 2016.
Ni-Cd cells are available in common sizes like AAA, AA, C, and D, and also as 9-volt battery equivalents. A fully charged Ni-Cd cell gives about 1.25 to 1.35 volts. This voltage stays quite steady as the battery is used. Since alkaline batteries can drop to 0.9 volts when nearly empty, Ni-Cd cells usually work well as replacements in most devices.
You can also find battery packs with many Ni-Cd cells, sometimes up to 300 cells (giving about 360 volts). These are used in cars and heavy industrial machines. For portable devices, packs usually have fewer than 18 cells (up to 24 V). Large industrial batteries can store hundreds of ampere-hours of power.
Ni-Cd vs. Other Batteries
Newer batteries like nickel-metal hydride (NiMH) and lithium-ion batteries are now common and cheaper. They can store more energy than Ni-Cd batteries. However, Ni-Cd batteries are still very useful when you need a lot of power very quickly, because they can handle high power use without being damaged.
Here are some advantages of Ni-Cd batteries compared to others:
- They are tough and hard to damage. They can even be fully discharged for a long time without harm. This is different from lithium-ion batteries, which can be permanently damaged if they run too low.
- They work very well in tough conditions, which is good for tools.
- Ni-Cd batteries usually last longer, meaning they can be charged and discharged many more times than batteries like lead-acid ones.
- They are smaller and lighter than lead-acid batteries that store the same amount of power. This is why they are often used in airplanes.
- Compared to regular alkaline batteries, Ni-Cd batteries can be reused many times, saving money in the long run.
- Their voltage stays steady as they discharge, which works well for most electronic devices.
- They can provide almost their full power even when you need a lot of current very fast. A lead-acid battery, for example, would only give about half its power in the same situation.
- Ni-Cd batteries can handle very high continuous power use, more than NiMH batteries.
The main downsides of Ni-Cd batteries are their higher cost and the use of cadmium. Cadmium is a toxic heavy metal, which means it's bad for the environment and living things. This is why they need special disposal. Also, Ni-Cd batteries can get hotter as they charge, which can be a problem for simple charging systems.
Memory Effect
Ni-Cd batteries can sometimes seem to have a "memory effect". This happens if they are repeatedly charged and discharged to the exact same level many times. The battery might then "remember" this point. When you use it again, the voltage might suddenly drop at that "remembered" point, making it seem like the battery is dead, even if it still has power. The battery's actual capacity isn't greatly reduced, but it can be annoying for devices.
This idea of a "memory effect" might have come from satellites that were charged and discharged in the same way over many years. It's unlikely that people using everyday electronics would cause this exact problem. The original research on this effect was later questioned by the scientists who wrote it.
You can often reduce this "memory effect" by fully discharging the battery about once a month. This helps the battery "forget" the specific charge point.
A similar problem is called voltage depression or lazy battery effect. This happens from charging the battery too much, too often. The battery seems full but runs out of power quickly. Sometimes, you can fix this by fully discharging and recharging the battery a few times. However, doing this too often can shorten the battery's life. If you take good care of a Ni-Cd battery, it can last for 1,000 charges or more.
Environmental Impact
Ni-Cd batteries contain cadmium, which is a toxic heavy metal. Because of this, they need to be disposed of carefully.
In the United States, the cost of recycling these batteries is often included in the price when you buy them.
In the European Union, a rule called the "batteries directive" (2006/66/EC) has banned the sale of Ni-Cd batteries for most everyday consumer devices. They are still allowed for medical use, alarm systems, emergency lighting, and some power tools (though the power tool use was also banned in 2016). Industrial Ni-Cd batteries must be collected and recycled by their makers.
See also
 In Spanish: Batería de níquel-cadmio para niños
In Spanish: Batería de níquel-cadmio para niños
- Battery recycling
- Comparison of battery types
- List of battery sizes
- List of battery types
- Power-to-weight ratio