Nuclear magnetic resonance spectroscopy facts for kids
Nuclear Magnetic Resonance spectroscopy, or NMR spectroscopy, is a cool scientific method. It helps scientists look at the tiny magnetic fields around the center (called the nucleus) of atoms. Think of it like a special camera that can see inside molecules!
NMR works by using radio waves and strong magnets. When a sample is put into a powerful magnetic field and then hit with radio waves, the nuclei inside the atoms respond in a special way. This response is called "nuclear magnetic resonance." Sensitive receivers then pick up these signals.
Each atom in a molecule has a slightly different magnetic field around it. This difference changes how the atom responds to the radio waves. By studying these signals, scientists can figure out the exact structure of a molecule. It's like getting a unique fingerprint for each chemical compound!
NMR is super important in organic chemistry. It's the best way to identify many different types of molecules. Biochemists also use NMR to study large molecules like proteins. Besides just identifying molecules, NMR gives detailed info about their shape, how they move, and what they're doing in chemical reactions.
The most common types of NMR look at hydrogen and carbon atoms. But it can be used for any sample that has atoms with a special property called spin.
Contents
How NMR Works
NMR usually involves three main steps:
- First, the tiny magnets inside the atoms (called nuclear spins) line up in a strong, steady magnetic field.
- Next, a small, quick burst of radio waves (an "RF pulse") gently pushes these lined-up spins out of alignment.
- Finally, scientists detect and study the radio waves that the atoms send out as they return to their original alignment.
What NMR Can Tell Us
NMR signals are very unique and clear for small molecules. Different groups of atoms in a molecule (called functional groups) give different signals. Even similar groups with slightly different neighbors give distinct signals. This makes NMR much better than older methods for identifying chemicals.
One downside is that you need a fair amount of a pure substance (about 2 to 50 milligrams). Also, the sample usually needs to be dissolved in a liquid. Analyzing solid samples needs special machines and might not give as clear results. NMR is also not great for seeing very fast chemical changes, as it takes a bit of time to get a reading.
More Advanced NMR
Scientists have developed more complex NMR methods. In two-dimensional NMR (2D NMR), the machine looks for signals that are connected. This helps identify which atoms are next to each other in a molecule. There are even 3D and 4D methods for very complex molecules!
Another cool technique is nuclear Overhauser effect (NOE) spectroscopy. This method helps figure out how close atoms are to each other in space. By measuring these distances, scientists can build a 3D model of a molecule.
NMR Machines
NMR machines are quite expensive. Universities often have them, but they are less common in private companies. A modern NMR machine can cost a lot of money, sometimes millions of dollars!
These machines use very strong superconducting magnets, which are cooled by liquid helium. The stronger the magnet, the clearer the results. There are also smaller, less expensive machines that use regular magnets. These are good for simpler tasks like checking samples quickly. Some are even small enough to sit on a lab bench!
History of NMR
The idea of NMR was first discovered by Isidor Isaac Rabi in 1944, and he won a Nobel Prize for it. Later, in the late 1940s and early 1950s, two groups of scientists, one led by Edward Mills Purcell at Harvard and another by Felix Bloch at Stanford, developed NMR into a useful spectroscopy technique. Purcell and Bloch shared the 1952 Nobel Prize in Physics for their work.
Basic NMR Techniques
Resonant Frequency
When certain atoms (like hydrogen or carbon-13) are placed in a magnetic field, they absorb radio waves at a specific frequency. This frequency depends on the strength of the magnetic field. For example, in a very strong 21 Tesla magnet, hydrogen atoms respond at 900 MHz. Scientists often refer to a magnet by the frequency at which hydrogen atoms resonate in it.
Preparing Your Sample
An NMR machine usually has a sample holder that spins inside a very strong magnet. It also has a radio-wave emitter and a receiver. The sample is typically put into a thin glass tube called an NMR tube. Spinning the sample helps to get clearer results.
Special Solvents
Most liquids (solvents) contain lots of hydrogen atoms. If you used a regular solvent, its signals would be so strong they would hide the signals from your sample. To avoid this, scientists use special deuterated solvents. In these solvents, most of the hydrogen atoms are replaced with deuterium (a heavier version of hydrogen) which doesn't interfere with the signals. The most common one is deuterochloroform (CDCl3).
Keeping the Field Steady
For NMR to work well, the magnetic field around the sample must be super steady and even. High-tech NMR machines use special "shims" to make the magnetic field incredibly uniform, down to tiny parts per billion! The machine also has a "lock" system that constantly checks and adjusts the magnetic field to keep it perfect during the experiment.
Getting the Spectrum
After the sample is zapped with a radio-frequency pulse, the atoms send back a very weak signal. This signal is processed using a mathematical trick called a Fourier transform to turn it into an NMR spectrum. A single measurement gives a weak signal, so scientists usually repeat the process many times and average the results to get a clear spectrum. For hydrogen atoms, this can take just a few minutes. For heavier atoms like carbon-13, it can take much longer, sometimes hours.
Chemical Shift
When an atom with spin is in a magnetic field, it can be in one of two energy states: spin up or spin down. The difference in energy between these states is very small, which is why strong magnets are needed. When radio waves with the right energy hit the sample, they can make atoms jump from the lower energy state to the higher one.
Even though all hydrogen atoms have the same basic magnetic properties, they don't all give signals at the exact same frequency. This is because the electrons around each atom create tiny local magnetic fields. These local fields "shield" the nucleus from the main magnetic field. How much shielding there is depends on the electron environment around the atom.
Scientists use a special scale called "chemical shift" to report these differences. It's measured in "parts per million" (ppm). For example, a hydrogen atom in an aldehyde group might have a chemical shift around 10 ppm, while a hydrogen in a hydrocarbon might be around 1 ppm. This difference tells scientists a lot about what kind of chemical group the atom is part of.
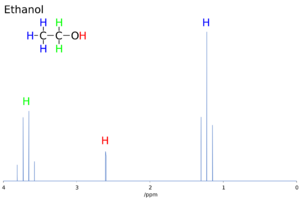
The chemical shift helps scientists figure out the structure of a molecule. For example, in ethanol (CH3CH2OH), you would expect three different signals: one for the CH3 group, one for the CH2 group, and one for the OH group. Each would appear at a different chemical shift.
The shape and size of the peaks in an NMR spectrum also give clues. The area under a peak is usually related to the number of atoms of that type in the molecule. So, if the CH3 peak in ethanol is three times bigger than the OH peak, it means there are three hydrogen atoms in the CH3 group for every one in the OH group.
J-coupling
| Multiplicity | Intensity ratio |
|---|---|
| Singlet (s) | 1 |
| Doublet (d) | 1:1 |
| Triplet (t) | 1:2:1 |
| Quartet (q) | 1:3:3:1 |
| Quintet | 1:4:6:4:1 |
| Sextet | 1:5:10:10:5:1 |
| Septet | 1:6:15:20:15:6:1 |
One of the most useful things in an NMR spectrum is something called J-coupling (or scalar coupling). This happens when the magnetic fields of atoms that are connected through chemical bonds affect each other. This interaction causes the NMR signals to split into multiple smaller peaks.
For example, if a hydrogen atom has one neighboring hydrogen atom, its signal will split into two peaks (a "doublet"). If it has two neighbors, it splits into three peaks (a "triplet"). The number of peaks and their intensity ratios follow a pattern called Pascal's triangle. This splitting pattern gives detailed info about how atoms are connected in a molecule.
In the ethanol example, the CH3 group is next to two CH2 protons, so its signal splits into a "triplet." The CH2 group is next to three CH3 protons, so its signal splits into a "quartet." This helps confirm the structure of the molecule.
J-coupling, along with chemical shift and peak area, tells scientists not only about the chemical environment of atoms but also about their neighbors within the molecule.
2D NMR: Seeing Connections
Correlation spectroscopy (COSY) is a type of 2D-NMR. It's like a map that shows which atoms are connected to each other. In 2D NMR, if two atoms are linked (either directly through bonds or by being close in space), a signal will appear on the map connecting their peaks.
2D NMR gives much more information than regular 1D NMR. It's super helpful for figuring out the structure of really complicated molecules, especially large ones like proteins. The first 2D NMR experiment was thought up in 1971 and put into practice in 1976.
Solid-State NMR
Sometimes, molecules can't be studied in a liquid solution, like in crystals or powders. For these solid samples, a special type of NMR called solid-state NMR is used. In solids, the atoms are fixed in place, which can make the NMR signals very broad and hard to read.
Scientists use special tricks to get clear signals from solids. One common method is called "magic angle spinning." This involves spinning the sample very, very fast (sometimes 20,000 times per second!) at a specific angle. This helps to sharpen the signals, making them similar to those from liquid samples.
Solid-state NMR is used to study things like membrane proteins, polymers, and even plant leaves!
Biomolecular NMR
Proteins
Protein NMR is a very important technique in structural biology. Scientists use it to figure out the 3D shapes of proteins. While X-ray crystallography is also used for this, NMR is often the only way to get detailed info about proteins that are flexible or don't have a fixed shape.
Proteins are much bigger than the small molecules we talked about earlier. Their NMR spectra can be very crowded. So, scientists use advanced 2D, 3D, or even 4D NMR experiments to sort out the signals. To make these experiments work better, proteins are often "labeled" with special versions of carbon (13C) and nitrogen (15N) atoms that are easier to see with NMR.
One key method for protein structure uses NOE experiments to measure distances between atoms. These distances are then used to build a 3D model of the protein. NMR can also show how different parts of a protein move and change shape.
Nucleic Acids
Nucleic acid NMR helps scientists learn about the structure and movement of DNA and RNA. In fact, many known RNA structures have been figured out using NMR!
Nucleic acid NMR is similar to protein NMR, but there are some differences. Nucleic acids have fewer hydrogen atoms, and their double helix shape means they don't fold up as much as proteins. Scientists use 2D NMR methods like COSY and NOESY to study them.
The info from NMR spectra helps determine local features like angles between parts of the molecule. For larger structures, these local details are combined with other models. NMR is great for looking at unusual shapes of DNA and RNA, and how they interact with other molecules like proteins or drugs.
Carbohydrates
Carbohydrate NMR helps scientists understand the structure and shape of carbohydrates (sugars). It can be tricky because many hydrogen atoms in carbohydrates give signals in a very similar range, making them hard to tell apart. However, special techniques help scientists investigate the different sugar units.
NMR in Drug Discovery
NMR can help scientists understand the shapes and movements of small molecules in a liquid. This is very useful in drug discovery. For example, it can show how a potential drug molecule changes its shape or interacts with other molecules. This information helps guide the design of new medicines.
NMR and Rechargeable Batteries
NMR is also used to study rechargeable batteries. Batteries are complex, with many different parts and surfaces where chemical reactions happen. These reactions are key to how batteries store and release energy.
Scientists use two main ways to study batteries with NMR:
- Ex situ NMR: Here, a part of the battery is taken out and put into the NMR machine. This method is great for getting clear signals from the solid parts of the battery, like the electrodes. It helps scientists see how the materials change as the battery charges and discharges.
- In situ NMR: In this method, the entire battery is placed inside the NMR machine. This is cool because it's non-destructive (the battery isn't opened), and scientists can watch what happens as the battery charges and discharges in real-time. This helps them find out about temporary changes that happen during battery operation.
|
 | Anna J. Cooper |
 | Mary McLeod Bethune |
 | Lillie Mae Bradford |