Zeise's salt facts for kids
Quick facts for kids Zeise's salt |
|
|---|---|
 |
|
 |
|
| IUPAC name | Potassium trichloro(ethylene)platinate(II) hydrate |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 630-445-1 |
| SMILES | [K+].C=C.Cl[Pt-](Cl)Cl.O |
|
InChI
InChI=1S/C2H4.3ClH.K.H2O.Pt/c1-2;;;;;;/h1-2H2;3*1H;;1H2;/q;;;;+1;;+2/p-3
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Yellow to orange crystals |
| Melting point | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
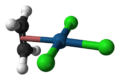
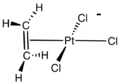
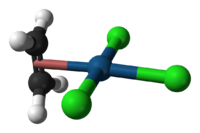
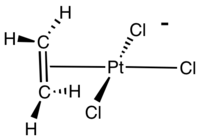
Zeise's salt is a special chemical compound that has the formula K[PtCl3(C2H4)]·H2O. It is also known by its longer name, potassium trichloro(ethylene)platinate(II) hydrate. This compound is usually a yellow color and is stable in the air.
It is a type of complex chemical, meaning it has a central metal atom (platinum) connected to other atoms or groups. In Zeise's salt, one of these groups is ethylene, which is a simple gas. The platinum atom in this salt has a flat, square shape around it.
Zeise's salt is very important in the history of organometallic chemistry. This is a field of chemistry that studies compounds with bonds between a metal and carbon atoms. Zeise's salt was one of the first examples of a compound where a metal was connected to an alkene (like ethylene). It is named after William Christopher Zeise, who discovered it.
Contents
How Zeise's Salt is Made
You can buy Zeise's salt already made, usually with water molecules attached to it.
To make it, chemists often start with a chemical called K2[PtCl4]. They mix it with ethylene gas. They also add a tiny amount of SnCl2, which helps the reaction happen faster. This helper is called a catalyst.
After it's made, any extra water can be removed using a special process that creates a vacuum.
What Zeise's Salt Looks Like Up Close
The ethylene part of Zeise's salt has a special bond between its carbon atoms, called a C=C bond. This bond is positioned almost straight up and down (perpendicular) to the flat part of the platinum and chlorine atoms.
In Zeise's salt and similar compounds, the ethylene group can spin around where it's connected to the metal. This spinning doesn't take much energy. Scientists have learned that the way the metal and ethylene are connected is a mix of two types of chemical bonds.
The Story of Zeise's Salt
Zeise's salt was one of the very first organometallic compounds ever found. It was discovered in 1830 by William Christopher Zeise. He was a professor at the University of Copenhagen.
Zeise was studying how a chemical called PtCl4 reacted with boiling ethanol. He carefully looked at what he made and thought that the new compound contained ethylene.
Another famous chemist at the time, Justus von Liebig, often disagreed with Zeise's idea. But in 1868, a scientist named Birnbaum made the same compound using ethylene, which proved Zeise was right!
Zeise's salt became very interesting to chemists in the late 1800s. They couldn't figure out its exact molecular structure. This mystery wasn't solved until the 20th century, when scientists used a method called X-ray crystallography to see its shape.
This discovery really pushed forward the field of organometallic chemistry. It helped scientists understand new ideas about how metals can connect to other molecules. The Dewar–Chatt–Duncanson model is one important idea that came from studying compounds like Zeise's salt. It explains how a metal connects to a C=C double bond.
Similar Compounds
There are other compounds that are like Zeise's salt:
- Zeise's dimer: This is made from Zeise's salt by taking away some parts and then joining two of the remaining pieces together.
- COD-platinum dichloride: This compound is also a platinum complex with an alkene. It's made from platinum(II) chloride and a chemical called 1,5-cyclooctadiene.
- Many other compounds with ethylene have been made. For example, ethylenebis(triphenylphosphine)platinum(0) has platinum in a different oxidation state (0 instead of II).
- Dichloro(ethylene)(α-methylbenzylamine)platinum(II): This is a special version of Zeise's salt. It's used to separate different forms of alkene molecules that are mirror images of each other.
Images for kids