Lauri Vaska facts for kids
Quick facts for kids
Lauri Vaska
|
|
|---|---|
| Born | May 7, 1925 Rakvere, Estonia
|
| Died | November 15, 2015 (aged 90) |
| Nationality | Estonia |
| Alma mater | Baltic University, University of Göttingen, University of Texas |
| Scientific career | |
| Fields | organometallic chemistry. |
| Institutions | Northwestern University Mellon Institute of Industrial Research (part of today's Carnegie Mellon University) Clarkson University |
Lauri Vaska (born May 7, 1925, in Rakvere, Estonia – died November 15, 2015) was an Estonian-American chemist. He made important discoveries in a field called organometallic chemistry. This area of chemistry studies special compounds that have a bond between a metal and a carbon atom.
Vaska studied at the Baltic University in Hamburg, Germany in 1946. He then went to the University of Göttingen from 1946 to 1949. There, he earned a degree similar to a Bachelor of Science. He later moved to the United States. From 1952 to 1956, he got his Ph.D. in inorganic chemistry at the University of Texas.
After that, Vaska worked at Northwestern University from 1956 to 1957. He researched how magnets affect chemicals there. In 1957, he became a Fellow at the Mellon Institute in Pittsburgh. He stayed there until 1964. Many famous chemists worked at the Mellon Institute during that time.
Vaska then became a professor at Clarkson University in Potsdam, New York. From 1990 until he passed away, he was a professor emeritus of chemistry. This means he was a retired professor who still kept his title. His brother, Vootele Vaska, was a philosopher. Lauri Vaska died in Basking Ridge, New Jersey in 2015, at 90 years old.
Amazing Discoveries in Chemistry
Lauri Vaska wrote about eighty articles for science journals. His work focused on the coordination chemistry of transition metals. These are metals like iron, copper, and gold. He also studied homogeneous catalysis, where chemicals help reactions happen faster. His research also covered organometallic and bioinorganic chemistry. Bioinorganic chemistry looks at how metals work in living things.
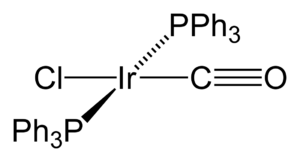
His time at the Mellon Institute was very productive. In 1962, with J.W. Di Luzio, he first described a special iridium compound. This compound became famous as Vaska's complex. Its full name is trans-IrCl(CO)[P(C6H5)3]2.
Vaska and his team showed that this iridium compound could react with small molecules. For example, it could add H2 (hydrogen gas) to itself. This process is called "oxidative addition." He also made another exciting discovery. His complex could reversibly bind to O2 (oxygen gas). This was a big deal at the time!
He discovered the main reactions of oxidative addition. This process is super important in homogeneous catalysis in organometallic chemistry. It helps chemists create new materials and make chemical reactions more efficient. He also showed how different parts of the molecule could change these reactions. For example, iridium(I) was more reactive than Rh(I). Also, adding iodide instead of chloride made the new compounds more stable.
Awards and Recognition
Lauri Vaska received several important awards for his work. In 1971, he won the Boris Pregel Award for Research in Chemical Physics. This award came from the New York Academy of Sciences.
In 1981, he was chosen as a Fellow of the American Association for the Advancement of Science. He received this honor for his "pioneering work in transition metal organometallic chemistry and synthetic oxygen carriers." This means he was recognized for his groundbreaking research on metal-carbon compounds and chemicals that could carry oxygen.
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |