Manganese dioxide facts for kids
Quick facts for kids Manganese dioxide |
|
|---|---|
 |
|
 |
|
| IUPAC name | Manganese oxide Manganese(IV) oxide |
| Other names | Pyrolusite, hyperoxide of manganese, black oxide of manganese, manganic oxide |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 215-202-6 |
| ChEBI | CHEBI:136511 |
| RTECS number | OP0350000 |
| SMILES | O=[Mn]=O |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Brown-black solid |
| Density | 5.026 g/cm3 |
| Melting point | |
| insoluble | |
| +2280.0·10−6 cm3/mol | |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−520 kJ·mol−1 |
| Standard molar entropy S |
53 J·mol−1·K−1 |
| Hazards | |
| EU classification | Harmful (Xn) Oxidizer (O) |
| NFPA 704 |
|
| R-phrases | R20/22 |
| S-phrases | (S2), S25 |
| Related compounds | |
| Other anions | Manganese disulfide |
| Other cations | Technetium dioxide Rhenium dioxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
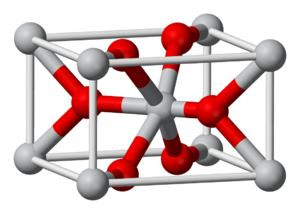
Manganese dioxide, also known as manganese(IV) oxide, is a common chemical compound made from manganese and oxygen. Its chemical formula is MnO2. This means it has one manganese atom and two oxygen atoms. It looks like a black, powdery solid.
Manganese dioxide is very important in many everyday items. It is often used inside batteries to help them work. You can also find it naturally as a mineral called pyrolusite.
Contents
What is Manganese Dioxide?
Manganese dioxide is a compound where manganese is in a special state called the +4 oxidation state. This state helps it react in different ways. It's a very stable compound, meaning it doesn't easily break down.
Where is it Found?
Manganese dioxide is found all over the world as the mineral pyrolusite. This mineral is a main source for getting manganese metal. Pyrolusite often forms in areas where water has helped minerals settle over time.
How is it Used?
Manganese dioxide has several important uses:
- In Batteries: It's used as a "depolarizer" in alkaline and Leclanche batteries. This means it helps prevent gases from building up inside the battery, which keeps the battery working longer and more efficiently.
- In Chemistry: It can be used in labs to make other chemicals. For example, it helps produce chlorine gas.
- In Glassmaking: Small amounts of manganese dioxide can be used to remove the green tint from glass, making it clearer. It can also be used to give glass an amethyst (purple) color.
- As a Pigment: Because of its dark color, it has been used as a pigment (a coloring agent) in paints and ceramics.
Properties of Manganese Dioxide
Manganese dioxide is a solid that is brown-black in color. It is quite dense, meaning a small amount of it weighs a lot. It does not dissolve in water. If heated to about 535 degrees Celsius (995 degrees Fahrenheit), it starts to break down.
Safety Information
Like many chemicals, manganese dioxide should be handled carefully. It is considered a harmful substance if swallowed or inhaled. It can also act as an oxidizer, which means it can help other materials burn. Always follow safety rules when working with chemicals.
Related pages
Images for kids
See also
 In Spanish: Óxido de manganeso(IV) para niños
In Spanish: Óxido de manganeso(IV) para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |