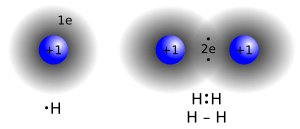Molecule facts for kids
A molecule is a tiny group of two or more atoms. These atoms are held together very strongly by special connections called chemical bonds. Think of them like tiny building blocks that link up to create everything around us!
Molecules can be made of just one type of atom. For example, the oxygen we breathe (O2) has two oxygen atoms. Or they can be made of different types of atoms. Water (H2O) is an example, with two hydrogen atoms and one oxygen atom.
Scientists have been curious about molecules for a long time. People like Robert Boyle, Amedeo Avogadro, and Linus Pauling helped us understand what molecules are and how they work. Today, we study them in fields like molecular physics and molecular chemistry.
Contents
- What's in a Name? The Word "Molecule"
- A Journey Through Time: Discovering Molecules
- The Science of Molecules
- Where Do We Find Molecules?
- How Atoms Stick Together: Chemical Bonds
- How Big (or Small!) Are Molecules?
- The Secret Language of Molecules: Formulas
- The Shape of Things: Molecular Geometry
- Listening to Molecules: Molecular Spectroscopy
- Thinking About Molecules: Theory
- Images for kids
- See also
What's in a Name? The Word "Molecule"
The word "molecule" comes from the Latin word "moles". This means a small unit of mass. The French word molécule also helped make it popular. It was first used in the late 1600s.
A Journey Through Time: Discovering Molecules
People have thought about tiny particles for thousands of years. Ancient Greek thinkers like Leucippus and Democritus believed everything was made of invisible "atoms" and empty space. Around 450 BC, Empedocles suggested that everything was made of four basic elements: fire, earth, air, and water. He also thought there were forces that made them attract or push away.
Later, in 1661, Robert Boyle had a new idea. He suggested that matter was made of "clusters of particles." He thought chemical changes happened when these clusters rearranged themselves.
In 1811, Amedeo Avogadro made a big step forward. He explained that the smallest particles of gases might not be single atoms. Instead, they could be groups of atoms joined together. He was the one who gave us the word "molecule"!
Many years later, in 1926, French physicist Jean Perrin proved that molecules really exist. He won the Nobel Prize for his work. He used different experiments to show how tiny particles move in liquids.
Then, in the 1930s, a scientist named Linus Pauling used new ideas from quantum mechanics. This helped him figure out how atoms connect to form molecules. He studied the angles and shapes of these connections. His work helped us understand the "nature of the chemical bond."
The Science of Molecules
Studying molecules is called molecular chemistry or molecular physics. Molecular chemistry looks at how molecules interact and form new bonds. Molecular physics focuses on their structure and properties.
A molecule is usually a stable system of two or more atoms. Sometimes, even electrically charged groups of atoms, called polyatomic ions, are thought of as molecules.
Where Do We Find Molecules?
Molecules are everywhere! They make up most of our oceans and the air we breathe. Most organic substances, which are important for life, are molecules. For example, proteins, DNA, sugars, and vitamins are all molecules.
However, not everything is made of individual molecules. Many common solid things on Earth are made of crystals or ionic compounds. These include all the minerals in rocks, sand, and even the Earth's core. These materials have many chemical bonds, but they don't have separate, identifiable molecules. Instead, their atoms are arranged in repeating patterns.
How Atoms Stick Together: Chemical Bonds
Molecules are usually held together by strong connections called covalent bonds. In these bonds, atoms share electrons. Imagine two friends sharing a toy; that's a bit like atoms sharing electrons! Many non-metallic elements, like hydrogen, exist as molecules in nature because their atoms are covalently bonded.
Covalent Bonds: Sharing is Caring
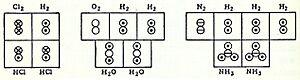
A covalent bond happens when two atoms share a pair of electrons. These shared electrons create a strong, stable connection between the atoms. For example, in a hydrogen molecule (H2), two hydrogen atoms share their electrons to form a bond.
Ionic Bonds: Giving and Taking Electrons
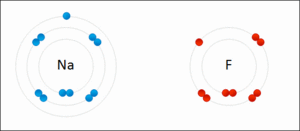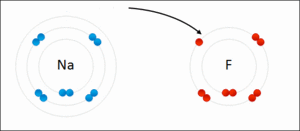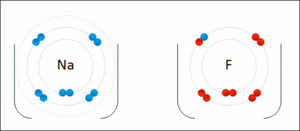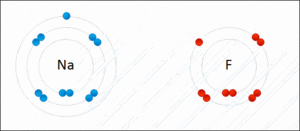
Ionic bonding is another type of chemical bond. It happens when atoms don't share electrons. Instead, one atom gives an electron to another. This creates oppositely charged particles called ions. The positive and negative ions then attract each other, like tiny magnets.
For example, when sodium and fluorine react, sodium gives an electron to fluorine. This forms sodium fluoride, which is a salt. Ionic bonds usually create solids, like table salt, where ions are arranged in a repeating pattern, not as separate molecules.
How Big (or Small!) Are Molecules?
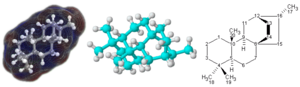
Most molecules are incredibly tiny. You can't see them with your naked eye. Some very large molecules, called polymers, can be big enough to see. DNA, for example, is a huge molecule!
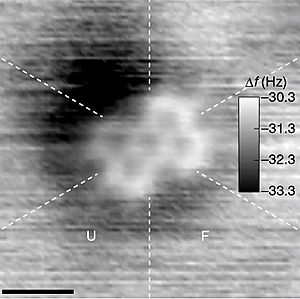
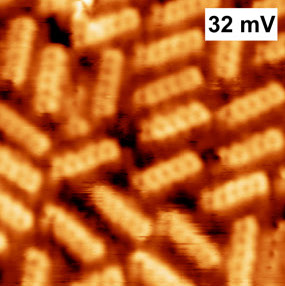
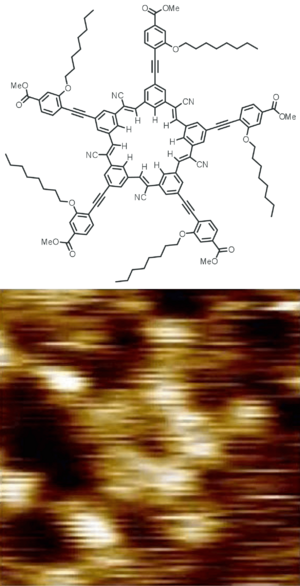
Typical molecules used in chemistry are only a few angstroms wide. An angstrom is about one billionth of a meter! While you can't see them with regular light, special tools like an atomic force microscope can help scientists see their outlines.
The smallest molecule is the hydrogen molecule (H2). It has a bond length of only 0.74 angstroms.
The Secret Language of Molecules: Formulas
Scientists use special codes called chemical formulas to describe molecules. These formulas use symbols for elements, numbers, and sometimes other signs.
Empirical Formulas: The Simplest Recipe
An empirical formula shows the simplest ratio of atoms in a molecule. For example, water always has two hydrogen atoms for every one oxygen atom (H2O). So, its empirical formula is H2O. Ethanol (alcohol) has carbon, hydrogen, and oxygen in a 2:6:1 ratio.
Molecular Formulas: The Exact Recipe
The molecular formula tells you the exact number of each type of atom in a molecule. For water, it's H2O. For acetylene, the molecular formula is C2H2. Its empirical formula would be CH, because that's the simplest ratio.
Sometimes, different molecules can have the same empirical formula but different molecular formulas. Or, they can even have the same molecular formula but different arrangements of atoms. These are called isomers.
Structural Formulas: Showing the Shape
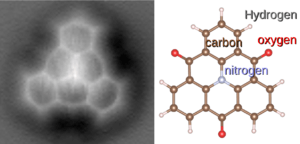
For molecules with complex 3D shapes, a simple chemical formula isn't enough. That's where a structural formula comes in handy. It's like a map that shows how all the atoms are connected and arranged in space.
The Shape of Things: Molecular Geometry
Every molecule has a specific, fixed shape. This shape includes the lengths of the bonds and the angles between them. Molecules are always wiggling and rotating, but they keep their average shape.
The chemical formula and the shape of a molecule are super important. They determine how a molecule will behave and react with other molecules. Think of it like a puzzle piece: only the right shape will fit!
Molecules with the same atoms but different arrangements (isomers) can have very different properties because their shapes are different.
Listening to Molecules: Molecular Spectroscopy
Spectroscopy is how scientists study molecules by "listening" to them. They send different types of energy, like light, at molecules. Then they observe how the molecules respond.
Molecules have specific energy levels. When they absorb or release energy, they create a unique "fingerprint" or spectrum. This helps scientists identify molecules and learn about their structure.
For example, infrared spectroscopy measures how molecules vibrate. This can tell us what kinds of bonds or groups of atoms are present. Microwave spectroscopy can measure how molecules rotate. It's even used to find molecules in outer space!
Thinking About Molecules: Theory
Scientists use quantum mechanics and computational chemistry to understand molecules even better. These advanced tools help them figure out how chemical bonds work.
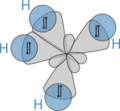
The simplest molecule studied this way is the hydrogen molecule-ion, H2+. It has two protons and one electron. This makes it easier to solve the complex math involved. With powerful computers, scientists can now study much more complicated molecules.
A molecule is considered stable if it has a deep enough "energy well" to hold its atoms together. This means it can exist for a certain amount of time. Even very weakly bound groups of atoms, like two helium atoms briefly sticking together, can be considered molecules under this idea.
Images for kids
See also
 In Spanish: Molécula para niños
In Spanish: Molécula para niños
- Atom
- Chemical polarity
- Chemical structure
- Covalent bond
- Diatomic molecule
- List of compounds
- List of interstellar and circumstellar molecules
- Molecular biology
- Molecular design software
- Molecular engineering
- Molecular geometry
- Molecular Hamiltonian
- Molecular ion
- Molecular modelling
- Molecular promiscuity
- Molecular orbital
- Non-covalent bonding
- Periodic systems of small molecules
- Small molecule
- Comparison of software for molecular mechanics modeling
- Van der Waals molecule
- World Wide Molecular Matrix