Robert H. Crabtree facts for kids
Quick facts for kids
Robert Crabtree
|
|
|---|---|

Robert Crabtree in London, February 2019
|
|
| Born |
Robert Howard Crabtree
17 April 1948 London, England, UK
|
| Nationality | British/United States |
| Education | Brighton College |
| Alma mater | University of Oxford (BA) University of Sussex (PhD) |
| Known for | Crabtree's catalyst |
| Awards | Corday-Morgan Prize (1982) Centenary Prize (2013) |
| Scientific career | |
| Fields | Organometallic chemistry |
| Institutions | Yale University Institut de Chimie des Substances Naturelles |
| Thesis | Transition Metal Dinitrogen Complexes Adduct Formation and Base Character (1973) |
| Doctoral advisor | Joseph Chatt |
| Other academic advisors | Malcolm Green Hugh Felkin |
Robert Howard Crabtree, born on April 17, 1948, is a famous British-American chemist. He is a professor at Yale University in the United States. Professor Crabtree is well-known for creating "Crabtree's catalyst". This special chemical helps add hydrogen to other substances. He also wrote an important textbook about organometallic chemistry.
Contents
Robert Crabtree's Education Journey
Robert Howard Crabtree went to Brighton College from 1959 to 1966. He then studied at the University of Oxford, earning a Bachelor of Arts degree in 1970. There, he learned from Malcolm Green. Later, he earned his PhD from the University of Sussex in 1973. His supervisor for this research was Joseph Chatt.
Robert Crabtree's Career in Chemistry
After getting his PhD, Robert Crabtree worked as a postdoctoral researcher. He joined Hugh Felkin's team near Paris, France. He worked there from 1973 to 1977.
In 1977, Crabtree became a professor at Yale University. He taught Inorganic Chemistry. He became a full professor in 1985 and retired in 2021. Now, he is an emeritus professor, meaning he is retired but still connected to the university.
Books and Publications by Robert Crabtree
Robert Crabtree has written and edited many important chemistry books. These books help students and scientists learn about different areas of chemistry.
- The Organometallic Chemistry of the Transition Metals (This book has been updated seven times!)
- He helped edit the Encyclopedia of Inorganic Chemistry.
- He was an editor for the New Journal of Chemistry.
- He is the editor-in-chief for Comprehensive Organometallic Chemistry III.
- He is also the editor-in-chief for the Encyclopedia of Inorganic Chemistry.
- He was on the board of editors for the journal Science.
- Chemistry of the Transition Metals (2009)
- Handbook of Green Chemistry – Green Catalysis (2009) (He wrote this with others.)
Awards and Honors for Robert Crabtree
Robert Crabtree has received many awards and honors for his work in chemistry. These awards show how important his discoveries and teachings are.
- He was named a Fellow of the Alfred P. Sloan Foundation (1981).
- He received the Corday-Morgan Medal from the Royal Society of Chemistry (1982).
- He received the Organometallic Chemistry Prize from the American Chemical Society (1991 and 1993).
- He received the Mack Award from Ohio State University (1994).
- He received the ISI Highly Cited Author Award (2000).
- He received the Bailar Medal at University of Illinois (2001).
- He received an ACS Green Chemistry Award (2009).
- He became a member of the National Academy of Sciences (2017).
- He was elected a Fellow of the Royal Society (FRS) in 2018.
Robert Crabtree's Research Discoveries
Robert Crabtree's research has led to many important discoveries in chemistry. He has focused on making chemical reactions more efficient and understanding how molecules interact.
Understanding Hydrogenation with Crabtree's Catalyst
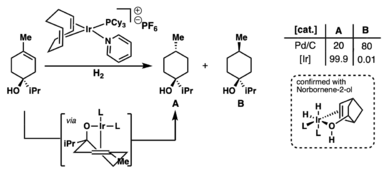
Robert Crabtree is famous for his work on hydrogenation. This is a chemical reaction that adds hydrogen to a molecule. He developed a special chemical called Crabtree's catalyst. This catalyst uses a metal called iridium. It is very good at adding hydrogen exactly where it's needed in a molecule.
For example, when a substance called terpinen-4-ol is hydrogenated, Crabtree's catalyst is 1000 times better at adding hydrogen to a specific part of the molecule. Other catalysts are not as precise. This precision is important for making specific chemicals.
Crabtree also studied how to reverse hydrogenation. This is called dehydrogenation, where hydrogen is removed. He showed how to remove hydrogen from a molecule called cyclooctane. This was one of the first times scientists showed how to activate C–H bonds using a metal complex. This discovery was a big step in his career.
Discovering New Types of Hydrogen Bonding
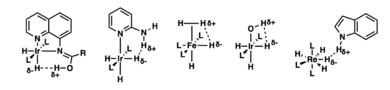
Another area of Crabtree's research is about a new kind of hydrogen bonding. Hydrogen bonds are like tiny magnets that hold molecules together. Usually, a hydrogen atom connects to an electronegative atom like oxygen.
Crabtree found that hydrogen can also bond in unusual ways. He discovered "dihydrogen bonds." In these bonds, two hydrogen atoms from different molecules get very close. These special bonds are much shorter than typical hydrogen-hydrogen distances. His findings help us understand how molecules are structured and how to design new catalysts.
Introducing Mesoionic Carbenes (MICs)
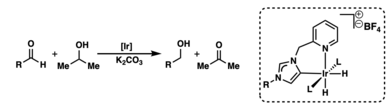
Crabtree has also made big contributions to the study of carbene chemistry. Carbenes are special carbon compounds. He explored "mesoionic carbenes" (MICs), also called "abnormal carbenes." These carbenes are useful as ligands. Ligands are molecules that attach to metal atoms in chemical reactions.
Crabtree found new ways to create and study these abnormal carbenes. He showed how they can be used with iridium in reactions that transfer hydrogen. This helps make chemical processes more efficient.
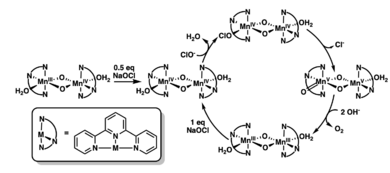
Studying Oxygen Production with Manganese
Crabtree's research also helps us understand how oxygen is made in nature. He studied special manganese compounds called di-μ-oxo dimers. These compounds are involved in oxygen evolution, which is the process of making oxygen.
He proposed a simple way these manganese compounds can produce oxygen. His work showed that the oxygen atoms in the new oxygen molecule come from water. This research helps us understand how plants make oxygen during photosynthesis.
Robert Crabtree's work has greatly advanced our knowledge in many areas of chemistry. He is known for choosing unique projects and developing new, stable catalysts. He also shares his knowledge through his writing, helping to educate future chemists.