Anethole facts for kids
Anethole, sometimes called anise camphor, is a special kind of organic compound that smells and tastes like anise or fennel. It's used a lot to add flavor to foods and drinks. You can find anethole naturally in the essential oils of plants like anise, fennel, liquorice, and star anise. It's what gives these plants their unique scent and flavor.
Anethole is usually a clear liquid that smells nice. It doesn't mix well with water, but it dissolves easily in ethanol (a type of alcohol). This is why some anise-flavored drinks turn cloudy when you add water to them – it's called the "ouzo effect"!
Quick facts for kids Anethole |
|
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
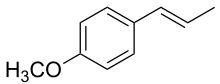
1-Methoxy-4-[(1E)-prop-1-en-1-yl]benzene
|
|
| Other names | (E)-1-Methoxy-4-(prop-1-en-1-yl)benzene (E)-1-Methoxy-4-(1-propenyl)benzene para-Methoxyphenylpropene p-Propenylanisole Isoestragole trans-1-Methoxy-4-(prop-1-enyl)benzene |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | D02377 |
| ChEBI | CHEBI:35616 |
| SMILES | O(c1ccc(\C=C\C)cc1)C |
|
InChI
InChI=1/C10H12O/c1-3-4-9-5-7-10(11-2)8-6-9/h3-8H,1-2H3/b4-3+
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Density | 0.998 g/cm3 |
| Melting point | |
| Boiling point | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Contents
How Anethole is Made
Anethole is a type of ether that has a special structure with a double bond. This double bond means it can exist in two different forms, called cis and trans isomers. The trans form is the one most commonly used.
Most anethole used today comes from extracts of trees, similar to turpentine. It can also be taken from the essential oils of plants, but this is less common for large-scale production.
Here's how much trans-anethole is found in some essential oils:
| Essential oil | World production (1999) | Trans-anethole |
|---|---|---|
| Anise | 8 tonnes | 95% |
| Star anise | 400 tonnes | 87% |
| Fennel | 25 tonnes | 70% |
Today, companies like Banwari Chemicals Pvt Ltd in India are major producers of anethole. They make it from other chemicals like 4-methoxypropiophenone.
What Anethole is Used For
Anethole has many interesting uses, especially because of its strong flavor and other properties.
Adding Flavor
Anethole is very sweet, about 13 times sweeter than regular sugar! People find its taste very pleasant, even in small amounts. It's a key ingredient in many alcoholic drinks like ouzo, rakı, anisette, and absinthe. You'll also find it in candies, chewing gum, toothpastes, and even in some natural berry flavors.
Fighting Germs and Pests
Scientists have found that anethole can fight against tiny living things like bacteria, yeasts, and fungi. It can stop them from growing or even kill them. For example, it can help against certain types of Salmonella bacteria. It also makes some other plant chemicals work better against yeasts.
Anethole is also a promising insecticide, meaning it can kill insects. It works against mosquito larvae and other pests like fungus gnats and mold mites. It can be used as a fumigant (a smoke or vapor that kills pests) or as a spray. It's even good at keeping mosquitoes away!
The Ouzo Effect

Anethole is famous for causing the "ouzo effect" (also called the "louche effect"). This happens when you mix drinks that contain anethole with water. The anethole doesn't dissolve well in water, so it forms tiny droplets that make the drink look cloudy or milky. This effect is not just cool to look at; it could also be useful in the food and medicine industries.
Is Anethole Safe?
In the USA, anethole is generally considered safe to use in food and drinks. There were some concerns in the past about whether it could harm the liver or cause cancer in rats, but studies have shown that it's very unlikely to cause these problems in humans when used as a flavoring.
Experts from the Joint FAO/WHO Expert Committee on Food Additives (JECFA) have looked at anethole closely. They found that in very large amounts, it could make animals less active, lower their body temperature, or have effects like a hypnotic (something that makes you sleepy) or painkiller. However, they concluded that there's "no safety concern at current levels of intake when used as a flavoring agent."
In very large quantities, anethole can be slightly toxic and might irritate the skin or eyes. But this is usually only if you come into contact with a lot of it.
A Brief History of Anethole
People have known for a long time that oils could be taken from anise and fennel plants. Alchemists and botanists in the Renaissance knew about this.
The chemical study of anethole began in 1820 with the Swiss chemist Nicolas-Théodore de Saussure. Later, in 1832, the French chemist Jean Baptiste Dumas figured out that the crystal-forming parts of anise oil and fennel oil were the same. He also worked out anethole's basic chemical formula.
In 1845, another French chemist, Charles Frédéric Gerhardt, gave it the name anethol. This name comes from the Latin words anethum (for anise) and oleum (for oil). Even though the correct structure of anethole was suggested in 1866, it wasn't fully accepted until 1872.
Images for kids
See also
 In Spanish: Anetol para niños
In Spanish: Anetol para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |