Aromatic compound facts for kids
Aromatic compounds are special organic compounds that contain one or more aromatic rings. Think of these rings as flat, stable circles of atoms. The word "aromatic" used to mean "smelly" because many of these compounds have strong smells. But now, the word describes their unique chemical structure, not their odor.
Heteroarenes are similar to aromatic compounds. They have at least one carbon atom in their ring replaced by another type of atom. These "other" atoms are often oxygen, nitrogen, or sulfur. For example, furan has a five-sided ring with an oxygen atom. Pyridine has a six-sided ring with a nitrogen atom. Compounds without an aromatic ring are called aliphatic.
Contents
What is a Benzene Ring?
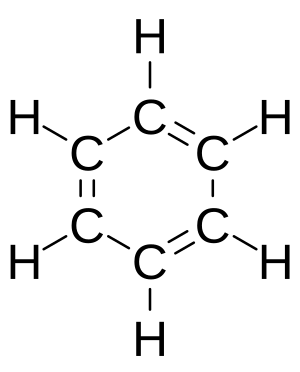
Benzene, with the chemical formula <chem>C6H6</chem>, is the simplest aromatic compound. It was the first one to be called "aromatic." A scientist named August Kekulé figured out its structure in the 1800s.
Imagine benzene as a flat, six-sided ring made of carbon atoms. Each carbon atom has four electrons it can share. One electron connects to a hydrogen atom. Two more connect to its two carbon neighbors. This leaves one electron for each carbon. These leftover electrons don't stay in one place. Instead, they float around the entire ring, above and below it. This makes all the bonds between the carbon atoms equally strong.
Sometimes, you'll see benzene drawn as a hexagon with a circle inside it. This circle shows that the electrons are spread out, or "delocalized," across the whole ring. This special arrangement makes aromatic compounds very stable.
Here are some general features of aromatic compounds:
- They have a special stability called aromaticity.
- They have many carbon atoms compared to hydrogen atoms.
- They often burn with a yellow, sooty flame because of all the carbon.
- They take part in specific chemical reactions where atoms are swapped.
The circle symbol for aromaticity was first used by Sir Robert Robinson in 1925. It helps scientists quickly understand the unique electron setup in these rings.
How Aromatic Compounds React
Aromatic rings are involved in many different chemical reactions.
Swapping Parts (Substitution)
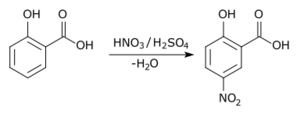
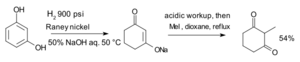
In a type of reaction called aromatic substitution, one part of the aromatic ring is replaced by another. Usually, a hydrogen atom is swapped out.
- If the new part is an "electrophile" (something that likes electrons), it's called electrophilic aromatic substitution.
- If the new part is a "nucleophile" (something that likes positive charges), it's called nucleophilic aromatic substitution.
Here's an example of an electrophilic substitution reaction:
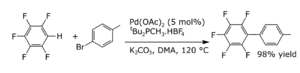
Joining Together (Coupling)
Coupling reactions are where two parts join together. Often, a metal helps this reaction happen. For aromatic compounds, coupling reactions can create new bonds between carbon atoms. They can also form new bonds between carbon and nitrogen, or carbon and oxygen.
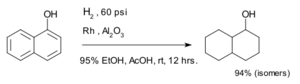
Adding Hydrogen (Hydrogenation)
Hydrogenation is a process where hydrogen atoms are added to an aromatic ring. This makes the ring "saturated," meaning it has as many hydrogen atoms as it can hold. It changes the aromatic ring into a non-aromatic, saturated ring.
Benzene and Its Family
Benzene can have other atoms or groups of atoms attached to its carbon ring. These are called "substituents."
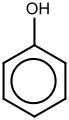
- Phenol is a benzene ring with a hydroxyl group (OH) attached.
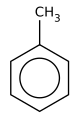
- Toluene is a benzene ring with a methyl group (CH3) attached.
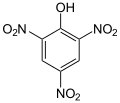
When there is more than one substituent on the ring, their positions matter. Scientists use special terms like ortho, meta, and para to describe where these groups are located on the ring. For example, cresol has a methyl group and a hydroxyl group. Depending on where they are, there are three different versions (isomers) of cresol.
- Some common aromatic compounds
The benzene ring is very good at stabilizing electric charges. This is why phenol, for example, can easily lose a hydrogen atom from its OH group, making it slightly acidic. The negative charge left behind on the oxygen atom can spread into the benzene ring, making it more stable.
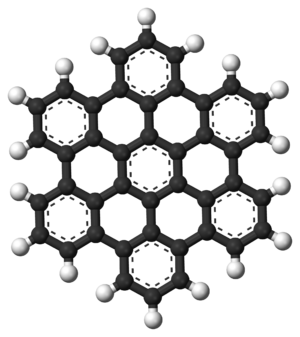
Polycyclic Aromatic Hydrocarbons (PAHs)
Polynuclear aromatic hydrocarbons (PAHs) are a type of aromatic compound that have two or more aromatic rings joined together. They don't have other atoms like oxygen or nitrogen in their rings. Naphthalene, which is found in mothballs, is the simplest example of a PAH.
PAHs are found naturally in oil, coal, and tar. They are also created when fuels like wood or gasoline are burned. You can even find PAHs in some cooked foods, especially meat cooked at high temperatures, like grilling or barbecuing, and in smoked fish.
Scientists are interested in PAHs because some of them can be harmful to living things. They can cause changes in cells that might lead to health problems.
Interestingly, PAHs are also found in space! They exist in the dust and gas between stars, in comets, and in meteorites. Some scientists even think PAHs might have played a role in the very first forms of life on Earth. In a material called graphene, the PAH structure is repeated over and over to form huge, flat sheets.
See Also
 In Spanish: Compuesto aromático para niños
In Spanish: Compuesto aromático para niños
- Aromatic substituents: Aryl, Aryloxy and Arenediyl
- Asphaltene
- Hydrodealkylation
- Simple aromatic rings
- Rhodium-platinum oxide, a catalyst used to hydrogenate aromatic compounds.
 | Bessie Coleman |
 | Spann Watson |
 | Jill E. Brown |
 | Sherman W. White |