Bromous acid facts for kids
Quick facts for kids Bromous acid |
|
|---|---|
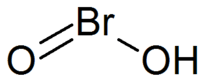 |
|
| IUPAC name | hydroxy-λ3-bromanone hydroxidooxidobromine bromous acid |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:29247 |
| SMILES | O=BrO |
|
InChI
InChI=1/BrHO2/c2-1-3/h(H,2,3)
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Related compounds | |
| Other anions | Hydrobromic acid; hypobromous acid; bromic acid; perbromic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Bromous acid is a special chemical compound. It is a type of acid. Its chemical formula is HBrO2. This means it contains hydrogen, bromine, and oxygen. In this compound, bromine is in a specific chemical state called its +3 oxidation state.
How Bromous Acid is Made
Bromous acid is not found naturally. Scientists have to make it in a lab. There are a few ways to create it:
- One way is to mix hypobromous acid with hypochlorous acid. This process is called oxidation, where one chemical helps another lose electrons.
- Another method involves a careful process called disproportionation. This is when a single substance changes into two different substances. In this case, hypobromous acid changes into bromous acid and another compound.
- It can also be made by combining hypobromous acid and bromic acid.
Uses of Bromous Acid
Bromous acid can be used in certain chemical reactions. For example, it can help to reduce permanganates into manganates. In chemistry, reduction is the opposite of oxidation. It means a substance gains electrons.
Related Acids
See also
 You can also find information about bromous acid in Spanish: Ácido bromoso para niños
You can also find information about bromous acid in Spanish: Ácido bromoso para niños
Black History Month on Kiddle
Prominent African-American Labor Activists
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Bromous acid Facts for Kids. Kiddle Encyclopedia.
