Deployment of COVID-19 vaccines facts for kids
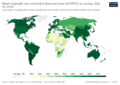
As of January 3, 2024, over 13.5 billion doses of the COVID-19 vaccine have been given out around the world. This means about 70.6% of people globally have received at least one dose. Even though many vaccines were given daily, by September 2022, only 22.3% of people in low-income countries had received their first vaccine. This shows a big difference in how vaccines were shared globally.
When the COVID-19 pandemic spread so quickly in 2020, groups like the World Health Organization (WHO) and the Coalition for Epidemic Preparedness Innovations (CEPI) worked with vaccine makers and governments to figure out how to get vaccines to everyone. Some worried that countries making vaccines might keep them for themselves or sell them to the highest bidder. Experts stressed that vaccines should be easy to get and affordable for healthcare workers and those who needed them most.
In April 2020, the UK agreed to work with 20 other countries, including France, Germany, and Italy, to find a vaccine and share the results. They promised that UK citizens would not get special treatment for vaccines developed with taxpayer money. Some companies planned to sell vaccines cheaply at first, then raise prices later if yearly shots were needed.
The WHO aimed to vaccinate 40% of people in all countries by the end of 2021 and 70% by mid-2022. However, many countries didn't reach the 40% goal.
Contents
How Vaccines Were Given Out
Many countries had plans to give out vaccines in phases. They usually started with people most at risk of getting very sick, like the elderly, and those most likely to be exposed, like healthcare workers.
Who Got Vaccines First?
In the United States, a group called the Advisory Committee on Immunization Practices (ACIP) decided in December 2020 that healthcare workers and people in nursing homes should get the first vaccines. The next group included people aged 75 and older, and essential workers like those in grocery stores, restaurants, law enforcement, fire departments, and schools. However, each state made its own final plans for how to give out the vaccines as more became available.
The European Union started its vaccine rollout on December 27, 2020. Each country managed its own distribution, but they all focused on healthcare workers, people at high risk, the elderly, and those with serious health problems.
The COVID‑19 vaccination programme in the United Kingdom first vaccinated people in elder care homes and their caregivers, then healthcare workers and those over 80. Later phases were mostly based on age, going down in 5-year steps.
Some countries gave out the first dose to as many people as possible, waiting longer for the second dose. This was done to protect more people quickly until more vaccines were ready. Studies also suggested that people who had already recovered from COVID-19 might only need one dose of an mRNA vaccine to get full protection.
Mixing Vaccine Doses
Using different vaccines for the first and second shots was not common. There wasn't much information on how well mixed vaccines worked, but experts didn't expect them to be unsafe. The US Centers for Disease Control and Prevention (CDC) suggested mixing vaccines only in special cases, like if a second dose of the same vaccine wasn't available soon.
In Canada, officials looked into mixing vaccines and eventually suggested using the Oxford-AstraZeneca COVID-19 vaccine for the first shot, followed by an mRNA vaccine. In June 2021, Germany recommended using mRNA vaccines as a second shot after an AstraZeneca shot for younger people. This was a safety step to avoid a rare blood clotting side effect linked to the AstraZeneca vaccine. Thailand also started mixing AstraZeneca and Sinovac vaccines in July 2021 due to worries about Sinovac's long-term protection.
Fair Access for Everyone
In 2020, as the COVID-19 pandemic grew, the World Health Organization (WHO) created a program called COVAX. Its motto was "No one is safe unless everyone is safe." This meant that vaccines needed to be shared fairly. COVAX aimed to provide COVID-19 vaccines to nearly 100 low-to-middle income countries that couldn't afford them. COVAX tried to raise money to buy and deliver vaccines to these countries based on their populations. By December 18, 2020, COVAX had agreements to get 1.3 billion doses for 92 low-to-middle income countries in the first half of 2021.
However, by mid-December, about 16 wealthy countries, which made up only 14% of the world's population, had ordered more than 10 billion vaccine doses. This was about 51% of all available vaccines. For example, Canada, Australia, and Japan, which had only 1% of the world's COVID-19 cases, had reserved about 1 billion vaccine doses. Meanwhile, COVAX had only reserved a few hundred million doses. At the 47th G7 summit in June 2021, the United States promised to give 500 million vaccine doses to other countries, and this started on August 17.
Wealthy countries ordered vaccines from 13 different companies in 2020. But low-to-middle income countries mostly ordered the Oxford–AstraZeneca COVID-19 vaccine because it was cheaper and didn't need special freezing.
Groups like CEPI, the WHO, and charities like the Gates Foundation raised over $20 billion in early 2020. This money was for developing vaccines and getting them ready, especially for children in developing countries. CEPI, which was created to make sure vaccines for infectious diseases were shared fairly, suggested thinking about how many vaccines could be made, how to pay for them, and protecting vaccine makers from legal problems. CEPI changed its policy to make sure its COVID-19 vaccine funding helped with fair access. This included:
- Keeping vaccine prices as low as possible for areas affected by outbreaks.
- Sharing information and knowledge about vaccine development with CEPI.
- Allowing CEPI to access and manage patents for promising vaccines.
- Giving CEPI a share of any money made from CEPI-funded vaccines to reinvest in public health.
- Sharing research results openly.
Other groups also pushed for fair access. Scientists encouraged working together to give out vaccines based on who was most at risk, like healthcare workers, vulnerable people, and children.
By mid-March 2021, 67 countries, mostly in Africa and the Middle East, had not yet started vaccinations. Countries that had started usually gave priority to health workers or the elderly. Some also suggested that people having surgery should get vaccinated because they might be more vulnerable. There were concerns about the short shelf-life of some vaccines, like Moderna and Pfizer-BioNTech. Once unfrozen, they had to be used within hours. So, some argued it was better to give these doses to anyone available rather than throw them away.
As of March 2021, the United States had ordered twice the number of doses needed for its own people. It was unclear when they would share extra doses. In April 2021, Vanity Fair reported that it would be hard to share extra doses because the U.S. government had agreed in its contracts to use vaccines only in the United States.
In November 2021, the World Health Organization said it was very important to fix the unfairness in vaccine access. They wanted to make sure vulnerable groups everywhere, like health workers and older people, got their first and second doses. They also said that unequal vaccine distribution helps new variants, like SARS-CoV-2 Omicron variant, appear.
Worries About Fairness
Some worried that wealthy countries would get their vaccines in 2020–21, while developing countries might not get them until 2023–24. Data from April 2021 showed this was happening: 25% of people in rich countries were vaccinated, compared to only 0.2% in poor countries.
On August 4, 2021, the head of the World Health Organization said that rich countries had given about 100 doses per 100 people, while poor countries had given only about 1.5 doses per 100 people. He believed it was important to vaccinate people in poor countries before giving booster shots in rich countries. The WHO Director General, Tedros Adhanom Ghedreyesus, worried that rich countries were keeping too many vaccines, leaving people in poorer nations waiting. The WHO's Africa office pointed out that some African countries had to throw away donated AstraZeneca vaccines because they were too close to expiring. Other countries also destroyed or paused shipments of vaccines due to expiration worries.
Wasted Doses
It's estimated that at least 215 million doses of COVID-19 vaccines bought by EU countries were thrown away by 2023.
Sharing Vaccine Information
The first polio vaccine was never patented, meaning its recipe was freely shared. Some argued that a similar approach for the COVID-19 vaccine could help share it fairly.
At first, talks at the World Trade Organization (WTO) about removing patent rights were blocked by countries like the US and the EU. But in April 2021, the US government changed its mind and announced support for a patent waiver on May 5, 2021. Many non-profit groups and European officials asked the US and Europe to support this idea.
The Debate About Patents
Some wondered if rules for sharing patents on simple drugs could apply to complex medicines like vaccines. One expert said that most people talking about removing patent protections had no experience in making vaccines. Most of the support for patent waivers came from public health groups, while vaccine experts often stayed quiet.
Simple drugs are easy to copy, and generic drug makers can quickly produce them without needing their own full tests. But there are "no such thing as generic vaccines." Each vaccine, even a copy, needs its own tests to prove it's safe and works. Copying an existing vaccine is so hard that the new version is often much better and can be patented itself.
Even if a patent is removed, a vaccine maker doesn't have to share all the secret knowledge (know-how) needed to make a vaccine. The WHO created a COVID-19 Technology Access Pool to help share this information, but vaccine makers didn't join. Without this know-how, trying to figure out how to make a vaccine is difficult and expensive, with no guarantee of success. Even if someone succeeds, they still need to prove it to health authorities. For simple drugs, proving a copy is similar costs about $1-2 million. But for complex medicines like vaccines, proving similarity requires new tests that can cost $100-250 million. One expert thought it would take at least two years after a patent waiver for the first copies of a COVID-19 vaccine to reach the market, which might be too late to help much.
Bill Gates said that "no free IP [intellectual property] would have improved anything related to this pandemic." His foundation helped other countries get deals to make vaccines, like the Oxford/AstraZeneca vaccine made by India's Serum Institute. Another worry was that allowing unauthorized companies to make vaccines would make it harder for original makers to get the raw materials they needed.
Because of these reasons, some believe that companies sharing their technology voluntarily is a better way to make more doses. This is because the original company can help the new one skip long testing processes. Others see patent waiver ideas as "more symbolic than practical." By November 2021, it seemed unlikely that patent waiver proposals would be approved because the WTO needs all members to agree. However, Tedros Adhanom Ghebreyesus said that both waiving patents and sharing technology were important steps to make more vaccines. He noted that the WTO's rules already allow for emergency waivers of intellectual property rights.
The debate about vaccine patents also involves who will control the future of new medicine technologies. Some believe that profits from strong patent rights are needed to encourage companies to spend a lot of money on research to turn lab ideas into real medicines. This research is very expensive (about $3 billion per successful drug) and often fails. If patents are waived, it might discourage companies from investing in new vaccines and life-saving technologies in the future. One expert said it would tell the industry: "Don't work on anything that we really care about, because if you do, we're just going to take it away from you." The worst outcome could be that drug companies stop focusing on saving lives and instead invent treatments for less serious conditions that are more profitable and less likely to be taken away.
Countries Keeping Vaccines for Themselves
Some countries wanted to keep vaccines for their own people first. This is called "vaccine sovereignty" or "vaccine nationalism." For example, the UK and the US made deals for the AstraZeneca-University of Oxford vaccine, raising concerns that these countries would get priority. The US paid $1.2 billion to get 300 million doses for Americans, even before the vaccine was proven safe or effective. There were worries that countries making vaccines might stop them from being exported to other nations.
In May 2020, the Chinese government promised that a successful Chinese vaccine would be a "global, public good," meaning enough doses would be made for both China and the rest of the world. Unlike some other vaccines, Chinese vaccines from Sinovac and Sinopharm don't need super-cold storage, making them easier to use in developing countries. In November 2021, China promised to donate 600 million more vaccine doses to Africa in 2022 and provide another 400 million through other ways.
In June 2020, the Serum Institute of India (SII), a big vaccine maker, agreed with AstraZeneca to make 1 billion vaccine doses for low- and middle-income countries. Half of these doses would go to India. Similar deals might mean that if a vaccine was made in Australia, Australians would get priority.
Unfair Ways to Get Vaccines
In the United States, healthcare workers and older people were high on the list for COVID-19 vaccination. But because it took a long time to get vaccines to everyone, some people tried to get ahead in line. They might try to bribe people or donate money to hospitals. Governments put in place big fines and other punishments for breaking these rules. A "black market" for COVID-19 vaccines also appeared, allowing some people to buy early, illegal access.
By mid-February 2021, China had arrested 80 people involved in illegal vaccine sales. The Colombian government stopped a traveler at an airport who had 70 doses of a Chinese vaccine without any proper documents.
Traveling for Vaccines
In early 2021, reports showed that wealthy people from Canada and Europe were flying to the United Arab Emirates to get vaccinated early. The UAE promoted Dubai as a place where rich people could pay a lot to get vaccinated before they were officially eligible in their home countries. Some Canadians who had second homes in the United States were also able to get vaccines earlier there.
As vaccine rules became less strict in the United States, wealthy people from other countries with slower vaccination rates reportedly traveled to the U.S. to get vaccinated. In April 2021, Alaska announced it would offer free vaccinations to tourists at its major airports starting June 1, 2021. To prevent this, Greece required a social security number for vaccination, but this also accidentally excluded some elderly people, immigrants, and Greek citizens who worked abroad.
In the European Union, some travel agencies started offering "vaccine vacations." The Maldives also offered vaccines as part of holiday travel packages.
How Much Vaccines Cost
An effective COVID-19 vaccine could save trillions of dollars globally. Some economists argued for temporarily removing patent protections for vaccines and paying the companies back. Any cost in the billions would seem small compared to the huge economic impact of the pandemic. In the early days, no one knew if a safe, reliable, and affordable vaccine could even be created, or how much it would cost. Even with several vaccines available, new variants of the virus meant that billions of dollars could still be invested without success.
Before a vaccine was developed, it was clear that billions of doses would need to be made and sent worldwide. In April 2020, the Gates Foundation thought manufacturing and distribution could cost as much as $25 billion. Bill Gates also said, "Ideally, there would be global agreement about who should get the vaccine first, but given how many competing interests there are, this is unlikely to happen." During development, 84–90% of vaccine candidates fail before final approval. Even in later stages, about 25.7% fail. This means a company might invest over $1 billion in a vaccine that never gets approved, leading to millions of useless doses. For the Oxford-AstraZeneca COVID-19 vaccine, 97% of its funding came from taxpayer money.
By November 2020, companies funded by the United States' Operation Warp Speed program set initial prices at $19.50 to $25 per dose, similar to the influenza vaccine. In December 2020, a Belgian politician accidentally revealed the secret prices agreed between vaccine makers and the EU. For example, the Oxford–AstraZeneca vaccine cost €1.78 per dose, while the Moderna vaccine cost €15.17 per dose.
Getting Vaccines Where They Need to Go
Getting COVID-19 vaccines to everyone needed a huge effort to transport and track 10–19 billion vaccine vials worldwide. This was one of the biggest supply chain challenges in history. By September 2020, experts worried that the global networks for distributing a vaccine weren't ready. This was partly because resources had been cut during the 2020 pandemic lockdowns. Globally, important supplies for vaccine research and development were becoming scarce due to international competition.
To handle this massive global challenge, Seth Berkley, chief executive of GAVI, said: "Delivering billions of doses of vaccine to the entire world efficiently will involve hugely complex logistical and programmatic obstacles all the way along the supply chain."
For example, the International Air Transport Association said that 8,000 Boeing 747 cargo planes, specially equipped for keeping vaccines cold, would be needed to transport just one dose for everyone in the more than 200 countries affected by COVID-19. GAVI stated that "with a fast-moving pandemic, no one is safe, unless everyone is safe."
While billions of dollars were invested in vaccine technology and early research, the plan for getting vaccines to people after they were approved didn't get as much attention or money. A big worry was that poor countries didn't have enough resources to distribute vaccines, especially for children. However, this could be improved by buying and distributing vaccines centrally for regions or countries. In September, the COVAX partnership included 172 countries working together to improve the vaccine supply chain. UNICEF joined COVAX to help with funding and supply for vaccinating children in 92 developing countries. By 2023, COVAX had provided over 1.6 billion doses to poor nations, helping to vaccinate 52% of their people, compared to a global average of 64%.
How Logistics Worked
Logistics services made sure that the necessary equipment, staff, and vaccines were available across countries. This included handling and monitoring vaccines, keeping them at the right cold temperatures, and making sure distribution was safe. The COVAX program aimed to centralize these logistics for all participating countries, bringing together manufacturing, transport, and the whole supply chain. This included tools for predicting vaccine needs, managing vaccines within countries, and handling waste.
Other important logistics steps for distributing COVID-19 vaccines internationally included:
- Using barcodes to track each vaccine vial.
- Sharing information about suppliers.
- Tracking where a vaccine vial came from, all the way from the factory to the person getting vaccinated.
- Using tools to monitor vaccine temperatures.
- Testing and ensuring temperature stability.
- Developing new packaging and delivery methods.
- Storing large amounts of vaccines.
- Coordinating supplies within each country (like personal protective equipment, syringes, needles, and power sources for refrigeration).
- Using communication technology.
- Considering environmental impacts in each country.
One vaccine developer said that a shortage in any one step could stop the whole supply chain. If the vaccine supply chain failed, the economic and human costs of the pandemic could last for years.
Making Enough Vaccines
By August 2020, even when only a few vaccine candidates were in late-stage trials and still months away from being proven safe and effective, many governments pre-ordered over 2 billion doses, costing more than $5 billion. The British government pre-ordered enough for five doses per person for 2021. This worried groups like the WHO and GAVI, who wanted fair access worldwide, especially for developing countries. By September, CEPI was supporting research for nine vaccine candidates and evaluating nine more. They aimed to make 2 billion doses of three approved vaccines by the end of 2021. Before 2022, 7–10 billion COVID-19 vaccine doses might be made worldwide. However, the large pre-orders by rich countries, sometimes called "vaccine nationalism," threatened vaccine availability for poorer nations.
The RNA vaccines from Moderna and Pfizer-BioNTech were especially hard to make because they used a new technology that had never been produced on such a large scale before. By February 2021, this was thought to be the main problem in making these vaccines. In November 2021, Moderna's CEO said that his company had millions of vaccine doses for Africa that couldn't be delivered because COVAX or individual governments couldn't accept them. He mentioned delays in giving out doses, not enough refrigerator space, and problems with customs documents.
Vaccines must be handled and transported according to international rules, kept at specific temperatures, and used before they spoil. The COVID-19 vaccine supply chain had to be huge to get vaccines to vulnerable people worldwide. Important steps for preparing facilities included having temperature-controlled areas and equipment, improving existing systems, training staff, and carefully monitoring everything. RFID technology was used to track and confirm vaccine doses from the factory all the way to the person getting vaccinated.
In September 2020, Grand River Aseptic Manufacturing agreed to help Johnson & Johnson make its vaccine. In October 2020, it was announced that the Moderna vaccine would be made in Switzerland by its partner Lonza Group, aiming to produce 300 million doses annually. The vaccine ingredients would be shipped frozen to Spain for the final manufacturing step. Lonza's site in New Hampshire aimed to make vaccine ingredients only for the U.S. by November 2020. Adding to the worries about rich countries' large pre-orders, manufacturing capacity was also limited because most vaccines were patented by companies in those countries. India and South Africa suggested a waiver to the TRIPS Agreement to allow new factories to be set up, but this was blocked by the G7 countries.
Keeping Vaccines Cold
Different vaccines needed different shipping and handling temperatures. For example, the Pfizer-BioNTech COVID‑19 vaccine had to be shipped and stored between -80 and -60 degrees Celsius (-112 and -76 degrees Fahrenheit). It had to be used within five days of thawing, and orders had to be for at least 975 doses. This made it hard to use in places other than large hospitals. The Moderna vaccine vials needed to be stored above -40 degrees Celsius (-40 degrees Fahrenheit) and between -25 and -15 degrees Celsius (-13 and 5 degrees Fahrenheit). Once refrigerated, the Moderna vaccine could be kept between 2 and 8 degrees Celsius (36 and 46 degrees Fahrenheit) for up to 30 days.
Vaccines are sensitive to temperature changes, so they need to be kept cold throughout the entire supply chain, usually between 2 and 8 degrees Celsius (36 and 46 degrees Fahrenheit). Because COVID-19 vaccines used different new technologies, there were new challenges for keeping them cold. Some vaccines were stable when frozen but sensitive to heat, while others shouldn't be frozen at all. If the cold chain temperature wasn't kept stable, the vaccine could be damaged and lose its effectiveness. Vaccines from Sinopharm and Sinovac, for example, could be transported using existing cold chain systems at 2–8 degrees Celsius.
Newer mRNA vaccine technologies were harder to make in large amounts and keep from spoiling. They needed very cold storage and transport. For example, Moderna's vaccine needed to be kept just above freezing (2–8 degrees Celsius) with limited storage time (30 days). But the Pfizer-BioNTech vaccine needed to be stored between -80 and -60 degrees Celsius (-112 and -76 degrees Fahrenheit) or colder until it was used. In February 2021, Pfizer and BioNTech asked the U.S. Food and Drug Administration (FDA) to allow their vaccine to be stored at -25 to -15 degrees Celsius (-13 to 5 degrees Fahrenheit) for up to two weeks before use. As of May 2021, another mRNA vaccine from Walvax was being tested and might be stored at room temperature for six months.
After a vaccine vial was opened to give a dose, it was only good for six hours and then had to be thrown away. This meant careful local management of cold storage and vaccination processes was needed. Because the COVID-19 vaccine would likely be in short supply in many places at first, vaccination staff had to avoid waste, which can be as much as 30% of the supply. The cold chain was also challenged by local transportation methods in rural areas, like motorcycles or delivery drones, the need for booster doses, and getting vaccines to vulnerable people like healthcare staff, children, and the elderly.
Transporting Vaccines by Air and Land
Coordinating international air cargo was very important for distributing COVID-19 vaccines quickly and at the right temperature. But by September 2020, the air freight network wasn't ready for such a big international effort. "Safely delivering COVID-19 vaccines will be the mission of the century for the global air cargo industry. But it won't happen without careful advance planning," said Alexandre de Juniac, IATA's Director General, in September 2020.
Because passenger air travel was greatly reduced in 2020, airlines cut staff, reduced destinations, and stored many planes. As the main groups for buying and supplying the COVID-19 vaccine through the WHO COVAX program, GAVI and UNICEF prepared for the largest and fastest vaccine distribution ever. This required international air freight cooperation, customs and border control, and possibly as many as 8,000 cargo planes to deliver just one vaccine dose to many countries.
Two of the first approved vaccines, Pfizer and BioNTech's Pfizer-BioNTech COVID‑19 vaccine and Moderna's mRNA-1273, had to be kept very cold during transport. This was done with special containers and dry ice. However, dry ice is only allowed in limited amounts on airplanes because the gases it releases can be harmful. In the United States, the Federal Aviation Administration (FAA) limited the amount of dry ice on a Boeing 777 to 3,000 pounds. But they temporarily allowed United Airlines to carry up to 15,000 pounds—nearly 1 million doses—between Brussels and Chicago. The Centers for Disease Control and Prevention (CDC) put McKesson Corporation in charge of vaccine distribution in the U.S., handling all major vaccines except Pfizer's. American Airlines, Boeing, and Delta Air Lines also worked to increase their dry ice transport capacity. FedEx and UPS installed ultra-cold freezers at air cargo hubs in Europe and North America, and UPS could make 1,200 pounds of dry ice per hour.
Safety and Security
Medicines are a huge target for fraud, making the high demand for a COVID-19 vaccine vulnerable to fake products, theft, scams, and cyberattacks throughout the supply chain. The vaccine was called "the most valuable asset on earth." Interpol warned of a "flood of all types of criminal activity." Steps were taken to prevent corruption and ensure transparency and accountability for COVID-19 vaccine supplies. If countries didn't have clear rules, good tracking, or ways to tell real vaccines from fake ones, it could be dangerous for people and make the pandemic last longer. Tracking technologies were used to trace vaccine vials and use digital tools to keep vaccination teams safe. In December 2020, Interpol warned that organized crime could get into the vaccine supply chain, steal products, or offer fake vaccine kits. Also, vaccines that needed constant freezing temperatures were at risk of being damaged on purpose.
GPS devices were used in the United States to track the vaccines. In Colorado, vaccine shipments were escorted by police officers from the airport to distribution points. The exact plans were kept secret.
An IBM security expert told The New York Times that hackers were targeting companies that make dry ice because it was so important for vaccine transport.
In May 2020, the FDA warned a company called North Coast Biologics that was selling a fake "nCoV19 spike protein vaccine." In January 2021, its founder, Johnny Stine, was arrested for selling mislabeled drugs. He pleaded guilty in August 2021 and was sentenced to five years of probation and ordered to pay back $246,986.
Country's Own Systems
The WHO created an "Effective Vaccine Management" system to help countries prepare their staff and facilities for vaccine distribution. This included:
- Trained staff to handle vaccines that are sensitive to time and temperature.
- Strong monitoring to ensure vaccines are stored and transported correctly.
- Facilities and equipment that can control temperature.
- Ways to track vaccines.
- Security measures.
Border processes for efficient handling and customs clearance within each country might include:
- Making it easier for planes to land.
- Allowing flight crews to skip quarantine.
- Making operations flexible for efficient national distribution.
- Giving arrival priority to keep vaccines at the right temperature.
Different Ways to Vaccinate
During a pandemic, there are two main goals: quickly vaccinating people who spread the virus a lot (socially active people) and vaccinating those most at risk (like the elderly, who are often less socially active). When there aren't enough vaccines, these goals can conflict. However, a 2022 study on vaccination plans in 29 countries (EU, UK, and Israel) showed that all plans prioritized people at higher risk (over 65 or with health conditions) over those who might spread the virus more (based on job or living conditions).
Some countries decided to delay the second vaccine dose so that more people could get their first dose faster. The first dose is more important for preventing severe illness. Using a smaller mRNA vaccine dose for younger people, who have a lower risk of severe disease but are key to spreading the virus, might allow more people to be vaccinated faster. Models predicted this could significantly reduce cases and deaths nationwide. On the other hand, some groups, like the elderly or those with weakened immune systems, might need extra booster doses. There were also worries about the impact of vaccination during pregnancy, partly due to misinformation on social media. This led to low vaccination rates in this group, even though studies showed COVID-19 vaccination had no harmful effects on live births or miscarriages.
Who is Responsible?
On February 4, 2020, the US Secretary of Health and Human Services announced a rule under the Public Readiness and Emergency Preparedness Act. This rule protected vaccine makers and healthcare providers from lawsuits about negligence, as long as there wasn't intentional wrongdoing. This rule is in effect in the United States until October 1, 2024.
In the European Union, COVID-19 vaccines were approved under a special Conditional Marketing Authorisation. This did not protect manufacturers from civil and administrative lawsuits. Even though the contracts with vaccine makers were secret, the makers were still responsible for side effects that weren't known when the vaccine was approved.
Pfizer was criticized for asking for very strong liability protections and other guarantees from countries like Argentina and Brazil. These demands went beyond what was expected from other countries like the US.
Images for kids
 | Bayard Rustin |
 | Jeannette Carter |
 | Jeremiah A. Brown |