Gas chromatography–mass spectrometry facts for kids
Gas chromatography–mass spectrometry (GC-MS) is a powerful tool that combines two different scientific methods: gas chromatography (GC) and mass spectrometry (MS). Together, they help scientists figure out exactly what chemicals are inside a sample, even if there are many different ones mixed together.
GC-MS is used in many important areas. For example, it helps investigate fires, check the environment for pollution, and find explosives. It's also great for identifying unknown substances. At airports, GC-MS can detect tiny amounts of substances in bags or on people, making travel safer. This technology can even find tiny traces of chemicals in very old or broken materials, when other tests might not work.
GC-MS is considered the best method for forensic experts to identify substances because it's a specific test. This means it can positively confirm the exact chemical present in a sample. A non-specific test, on the other hand, only tells you that a general type of substance is there. Relying only on non-specific tests could sometimes lead to wrong identifications.
Contents
How GC-MS Was Developed
The first ideas for gas chromatography appeared in 1950. Scientists used different detectors to see chemicals leaving the chromatograph. Most of these detectors would destroy the chemicals, often by burning them. This made it hard for chemists to know exactly what each chemical was.
In the 1950s, two scientists named Roland Gohlke and Fred McLafferty came up with a new idea. They used a mass spectrometer as the detector for gas chromatography. These early machines were large and delicate. They were mostly used only in special laboratories.
The first designs were quite complicated. It was tricky to control when different chemicals would come out of the chromatograph. The mass spectrometer had to finish analyzing one chemical before the next one arrived. In those early days, the results from the mass spectrometer were drawn on graph paper. Highly trained chemists then studied these patterns to identify each chemical.
By the 1970s, computers started to help. Special devices called analog-to-digital converters were added to mass spectrometers. This allowed computers to store and understand the results. As computers became faster and smaller, GC-MS machines also became quicker and more common. Today, computer-controlled GC-MS systems are widely used. They help monitor water, air, and soil for pollution. They are also used to check food safety and help in making medicine.
The invention of small computers made GC-MS machines much simpler. It also greatly sped up how long it takes to test a sample. In 1964, a company called Electronic Associates, Inc. (EAI) started working on a computer-controlled mass spectrometer. By 1966, they had sold over 500 gas-analyzer machines. In 1967, the Finnigan Instrument Corporation (FIC) was formed. They delivered the first working GC-MS machines to universities in 1968. FIC later became Finnigan Corporation and became a world leader in GC-MS systems.
How GC-MS Works Simply
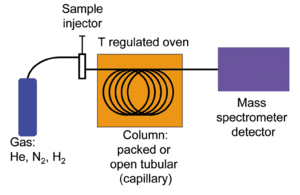
GC-MS can find all the different chemicals mixed together in a sample. First, a scientist dissolves the sample in a liquid. Then, this liquid is injected into a stream of gas, usually Helium, Hydrogen, or Nitrogen.
This gas flows through a long, thin tube that has a special coating inside. Each chemical in the sample sticks to this coating differently. Because of this, each chemical comes out of the tube at a different time. This process separates all the chemicals that were mixed together.
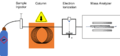
As each chemical comes out of the tube, it gets an electric charge (it becomes ionized). Most chemicals break into smaller pieces when they are ionized. These charged pieces then fly under a magnet. The magnet separates the pieces based on their weight and charge.
A computer then measures all the pieces from each chemical. By comparing these measurements to a huge computer library of known chemicals, the computer can identify all the chemicals in the sample. It can also tell you how much of each chemical was present.
Parts of the GC-MS Machine
The GC-MS machine has two main parts: the gas chromatograph and the mass spectrometer.
The gas chromatograph uses a very thin tube called a capillary column. The way this column separates chemicals depends on its size (length, width) and the special coating inside it. Different chemicals in a mixture will travel through the column at different speeds. This means they come out of the gas chromatograph at different times. This time is called the "retention time."
Once a chemical comes out of the gas chromatograph, it goes into the mass spectrometer. The mass spectrometer takes the chemical, gives it an electric charge, and breaks it into tiny pieces. It then measures these pieces based on their weight and charge.
Using these two machines together makes identifying chemicals much more accurate than using either one alone. It's very hard to identify a specific chemical with just gas chromatography or just mass spectrometry. The mass spectrometer usually needs a very pure sample. In the past, gas chromatography used other detectors that couldn't tell apart different chemicals that came out of the column at the same time. This could confuse the computer.
Sometimes, two different chemicals can also have very similar patterns of pieces in a mass spectrometer. But it's extremely unlikely that two different chemicals will act the same way in both a gas chromatograph and a mass spectrometer. So, if the mass spectrometer identifies a chemical, checking its retention time from the gas chromatograph makes scientists even more sure about what they've found.
What GC-MS Is Used For
Checking the Environment
Many scientists believe that GC-MS is the best tool for checking for harmful chemicals in the environment. The cost of GC-MS machines has gone down, and they have become more reliable. Both of these changes have led to more use in environmental studies. While some chemicals, like certain pesticides, are too similar to others to be identified by GC-MS, for most environmental samples, GC-MS is very sensitive and works well.
Helping Law Enforcement
GC-MS is used to detect illegal narcotics. It might even replace drug-sniffing dogs in some cases. It's also commonly used in forensic toxicology. This helps find drugs or poisons in samples taken from people involved in investigations.
Airport Security
After the terrorist attacks on September 11, 2001, explosive detection systems became a standard part of all U.S. airports. Many of these systems use GC-MS technology. Only a few companies are approved to provide these systems, and their machines often rely on GC-MS to find explosives.
Space Exploration
Several GC-MS machines have traveled into space! Two went to Mars as part of the Viking program. The Venera and Pioneer Venus missions used GC-MS to study the atmosphere of Venus. The Huygens probe landed a GC-MS on Saturn's largest moon, Titan. The Rosetta mission also used a special type of GC-MS to analyze material from the comet 67P/Churyumov-Gerasimenko in 2014.
Medical Uses
GC-MS is used in newborn screening tests. These tests can find many inherited metabolic diseases. These are conditions where the body has trouble breaking down certain substances. GC-MS can find tiny amounts of unusual chemicals in a baby's urine. These chemicals are usually not there but appear in babies with these disorders. This helps doctors diagnose these conditions early, so treatment can start sooner, leading to better health outcomes for the baby. It's now possible to test a newborn for over 100 genetic metabolic disorders using a urine test based on GC-MS.
GC-MS is also used with special "labeled" chemicals to study how the body's metabolic activity works. This often involves using 13C as a label and measuring the ratio of 13C to 12C with a special machine called an isotope ratio mass spectrometer (IRMS).
Images for kids
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |