Hermann Staudinger facts for kids
Quick facts for kids
Hermann Staudinger
|
|
|---|---|
 |
|
| Born | 23 March 1881 |
| Died | 8 September 1965 (aged 84) |
| Alma mater | Technische Universität Darmstadt, University of Halle |
| Known for | Ketenes Polymer chemistry Staudinger coupling Staudinger reaction Staudinger synthesis |
| Spouse(s) | Magda Staudinger (née Woit) |
| Awards | Nobel Prize in Chemistry (1953) Rudolf Diesel Medal (1962) |
| Scientific career | |
| Fields | Organic and Polymer chemistry |
| Institutions | University of Strasbourg University of Karlsruhe ETH Zürich University of Freiburg |
| Thesis | Anlagerung des Malonesters an ungesättigte Verbindungen (1903) |
| Doctoral advisor | Daniel Vorländer |
| Doctoral students | Werner Kern Tadeusz Reichstein Leopold Ružička Rudolf Signer |
Hermann Staudinger (23 March 1881 – 8 September 1965) was a German organic chemist. He is famous for showing that very large molecules, called macromolecules, exist. He also explained that these are made of smaller units linked together, which he called polymers. For this important work, he won the Nobel Prize in Chemistry in 1953.
Staudinger also discovered a group of molecules called ketenes. He is also known for the Staudinger reaction, a special chemical process. In the 1920s, he worked with Leopold Ružička to figure out the structures of natural chemicals called pyrethrins. This helped create modern insecticides in the 1960s and 1970s.
Contents
Early Discoveries
Hermann Staudinger was born in Worms, Germany, in 1881. He first wanted to study plants, but he chose chemistry instead. He studied at several universities, including the University of Halle and Technische Universität Darmstadt. In 1903, he earned his Ph.D. from the University of Halle.
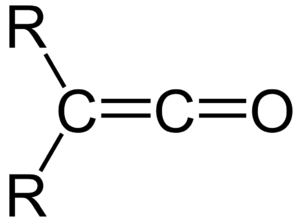
In 1907, Staudinger became a lecturer at the University of Strasbourg. Here, he discovered ketenes. These are a family of molecules with a special structure, as you can see in Figure 1. Ketenes later became very important for making antibiotics like penicillin. His wife, Dora Staudinger, helped him by writing down his lectures.
Later in 1907, Staudinger moved to the University of Karlsruhe. There, he successfully isolated many useful organic compounds. He also guided two future Nobel Prize winners, Leopold Ružička and Tadeusz Reichstein, in their studies.
The Staudinger Reaction
In 1912, Staudinger started a new job at the Swiss Federal Institute of Technology in Zurich, Switzerland. One of his first big discoveries there was in 1919. He and his colleague Meyer found that certain organic compounds, called organic azides, react with another chemical, triphenylphosphine. This reaction creates a new compound called an iminophosphorane.
This chemical process is now known as the Staudinger reaction. It is very useful because it usually produces a lot of the desired iminophosphorane. You can see how this reaction works in Figure 2.
Staudinger During World War I
During World War I, many German professors supported the war. However, Staudinger chose not to sign a public statement supporting it. He was one of the few who spoke out against the war, like Albert Einstein.
In 1917, Staudinger wrote an essay. In it, he predicted that Germany would lose the war because other countries had stronger industries. He called for peace as soon as possible. After the United States joined the war, he repeated his call for peace in a letter to the German military leaders. Another famous chemist, Fritz Haber, criticized Staudinger for his views. Haber accused him of harming Germany. Staudinger, in turn, criticized Haber for his role in developing chemical weapons for Germany.
Understanding Polymers
While working in Karlsruhe and Zurich, Staudinger began studying rubber. Scientists knew rubber had a very high molecular weight. At that time, many chemists believed that these high weights were just small molecules clumping together.
But in 1920, Staudinger suggested a new idea in a very important paper. He proposed that rubber and other materials like starch, cellulose, and proteins are actually very long chains. These chains are made of small, repeating units linked together by strong chemical bonds called covalent bonds. He said polymers are like chains of paper clips, where small parts are linked end-to-end (see Figure 3).
At first, many leading chemists did not believe Staudinger. They thought it was impossible for small molecules to link up covalently to form such huge compounds. This was partly because how molecules were structured and bonded was not fully understood back then.
In 1926, Staudinger became a chemistry professor at the University of Freiburg in Germany. He stayed there for the rest of his career. In 1927, he married Magda Voita, a botanist from Latvia. She worked with him until his death, and he recognized her help when he accepted his Nobel Prize.
More proof for Staudinger's polymer idea came in the 1930s. Scientists confirmed the high molecular weights of polymers using new methods. Staudinger also measured how thick polymer solutions were, which supported his theory. Studies using X-ray crystallography showed direct evidence of long chains of repeating units. Also, chemists like Wallace Carothers showed that polymers like nylon and polyester could be made using known chemical reactions.
Staudinger's theory completely changed how scientists understood these materials. It helped create the field of polymer science, which is now a very important part of chemistry.
Private Life
Hermann Staudinger first married Dora Förster in 1906. They had four children together. Their daughters, Eva Lezzi and Klara Kaufmann, were active in resisting the rise of fascism in Germany. Hermann and Dora divorced in 1926. Dora later remarried and became a well-known peace activist.
Legacy
Hermann Staudinger's amazing work helped us understand what high-molecular weight compounds, which he called macromolecules, really are. This paved the way for the entire field of polymer chemistry. Staudinger saw the huge potential of this science long before others did.
In 1936, he said, "It is not unlikely that sooner or later a way will be found to make artificial fibers from synthetic high-molecular products, because the strength and stretchiness of natural fibers depend only on their macro-molecular structure – that is, on their long thread-shaped molecules."
Staudinger started the first journal focused on polymer chemistry in 1940. In 1953, he received the Nobel Prize in Chemistry for his "discoveries in the field of macromolecular chemistry." In 1999, his work was recognized as an International Historic Chemical Landmark. His pioneering research has given the world countless plastics, fabrics, and other materials. These make everyday products more affordable and enjoyable, and help engineers build lighter and stronger structures.
See also
 In Spanish: Hermann Staudinger para niños
In Spanish: Hermann Staudinger para niños
- Beta-lactam
- Carbene
- Hypervalent molecule
- Polyoxymethylene
- Pyrethrin
- Triphenylphosphine phenylimide
- Heidegger and Nazism: denounced or demoted non-Nazis
 | Laphonza Butler |
 | Daisy Bates |
 | Elizabeth Piper Ensley |