Indigo dye facts for kids
Indigo is a special blue color that comes from a natural dye. It's a natural dye, meaning it comes from plants, especially the Indigofera plant, like Indigofera tinctoria. For a long time, indigo was one of the only ways to get a true blue color, making it very important around the world, especially in Asia.
Today, most indigo dye is made in factories, not from plants. About 80,000 tons are made each year! You probably see indigo every day, because it's used to dye denim cloth for your blue jeans. It's also why jeans can have cool looks like stone washing and acid washing.
Contents
What Indigo is Used For
The main use for indigo is to dye cotton yarn, which is then woven into denim fabric for blue jeans. A single pair of blue jeans needs about 3 to 12 grams of this dye. Smaller amounts are also used to dye wool and silk.
There's also a related dye called Indigo carmine. It's used as a colorant too, especially for blue jeans. It's even approved as a food colorant in the United States, known as FD&C Blue No. 2.
The Amazing History of Indigo
Indigo has a very long history! The oldest known fabric dyed with indigo was found in Peru and is about 6,000 years old. Many countries in Asia, like India, China, and Japan, have used indigo for centuries, especially for dyeing silk. Ancient civilizations in places like Mesopotamia, Egypt, and Peru also knew about this dye.
India was a major center for growing the Indigofera tinctoria plant and making indigo dye. From India, indigo traveled to the Greeks and Romans, who thought it was a very fancy product. The Greek word for the dye, indikón, meant "Indian," which shows how closely it was linked to India. This word later became "indigo" in English.
In ancient Mesopotamia, a clay tablet from about 700 BC even had a recipe for dyeing wool blue using indigo! The Romans used indigo for painting, medicine, and even cosmetics. It was a luxury item brought to the Mediterranean Sea by Arab traders.
In the 1800s, there was a huge demand for indigo. In 1897, about 7,000 square kilometers of land were used to grow indigo plants, mostly in India. That's a lot of land, much bigger than the country of Luxembourg!
For a long time, indigo was hard to get in Europe. People there used a similar dye from a plant called woad. But in the late 1400s, the Portuguese explorer Vasco da Gama found a sea route to India. This made it much easier and cheaper to bring indigo to Europe. Many European countries then started growing indigo plants in their colonies in warm places like Haiti and Jamaica. Sadly, much of this work was done by enslaved people. Some European countries, like France and Germany, even tried to ban imported indigo to protect their local woad dye businesses.
Because indigo was so valuable, it was often called "blue gold."
In West Africa, indigo was super important for textile traditions that were centuries old. From the Tuareg nomads in the Sahara desert to people in Cameroon, clothes dyed with indigo showed wealth. Women often dyed the cloth, and the Yoruba of Nigeria and the Mandinka of Mali were especially skilled. The Hausa male dyers in the city of Kano became very rich from their communal dye pits, which you can still see today. The Tuareg people are sometimes called the "Blue People" because the indigo dye from their traditional robes and turbans would stain their skin dark blue.

In Japan, indigo became very important during the Edo period. This was because the textile industry was growing, and ordinary people were not allowed to wear silk. So, cotton became popular, and indigo was one of the few dyes that worked well on cotton.
In North America, indigo was brought to colonial South Carolina by Eliza Lucas. It quickly became the second most important crop there, after rice. Like in other places, growing indigo in South Carolina relied on enslaved African and African American labor. By 1775, South Carolina was producing over 1.2 million pounds of indigo! When Benjamin Franklin went to France in 1776 to ask for help in the American Revolutionary War, he brought 35 barrels of indigo to sell to help pay for the war.
Making Indigo in Factories
In 1865, a German chemist named Adolf von Baeyer started trying to make indigo in a lab. He succeeded in 1878 and again in 1880. It took a few more years, but eventually, scientists figured out how to make indigo on a large scale in factories.
By 1897, a company called BASF had a way to make indigo that was cheap enough to sell widely. Before this, 19,000 tons of indigo came from plants. But by 1914, that number dropped to only 1,000 tons because factory-made indigo was so much easier to get. By 2011, about 50,000 tons of factory-made indigo were produced around the world each year.
How Indigo Dyes Fabric
Indigo is a tricky dye because it doesn't dissolve in water easily. To make it work, it has to go through a chemical change to become "white indigo," which can dissolve. When you dip fabric into the dye bath and then pull it out, the white indigo quickly mixes with the air and turns back into the bright blue, insoluble indigo.
When indigo first became popular in Europe in the 1500s, dyers had a hard time with it. It needed many chemical steps, some with dangerous materials, and workers could get hurt. In the 1800s, the poet William Wordsworth wrote about the tough conditions of indigo dye workers in his hometown.
One old way to make indigo dissolve was to use stale urine, which contains a chemical called ammonia. Another method, used in Japan, involved dissolving indigo in a hot vat with special bacteria. These bacteria helped turn the insoluble indigo into soluble white indigo. Fabric dyed this way was often decorated using techniques like shibori (tie-dye) and kasuri.
Printing with Indigo
Two ways to print directly with indigo were developed in England in the 1700s. One method, called 'pencil blue', used a brush to apply the dye. It could make very dark blues. The other method, 'China blue', made sharp designs like Chinese blue-and-white pottery. This method involved printing the insoluble indigo onto the fabric, then dipping it in different chemical baths.
Around 1880, the 'glucose process' was invented. This finally made it possible to print indigo directly onto fabric easily and cheaply, creating dark indigo prints.
Since 2004, you can even buy "freeze-dried indigo." This indigo has already been chemically changed, then frozen and dried into crystals. You just add the crystals to warm water to make a dye bath. It's very easy to use, and the crystals can be stored for a long time.
What Indigo is Made Of
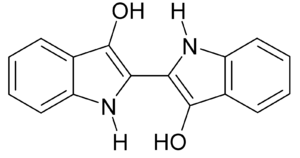
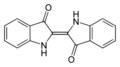
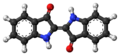
Indigo dye is a dark blue powder that can turn directly into a gas when heated to about 390-392 degrees Celsius. It doesn't dissolve in water, alcohol, or ether, but it can dissolve in some other liquids like chloroform. The chemical formula for indigo is C16H10N2O2.
The molecule gets its deep blue color because of how its chemical bonds are arranged, allowing it to absorb orange light.
Indigo's Relatives
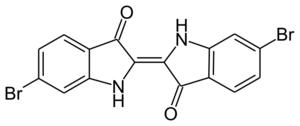
Scientists can change the indigo molecule a bit to create other dyes. For example, Tyrian purple is a dull purple dye that comes from a type of snail found in the Mediterranean Sea. It was very valuable in ancient times. Another related dye is Ciba blue.
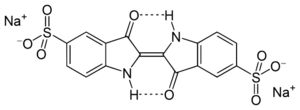
When indigo is treated with sulfuric acid, it turns into a blue-green dye called indigo carmine. This dye became available in the mid-1700s and is used to color food, medicines, and cosmetics.
Safety and the Environment
Indigo is not very harmful if swallowed. However, like any chemical, it should be handled carefully. In 2009, there were reports of large spills of blue dyes from a blue jeans factory in Lesotho, which raised environmental concerns.
See also
- Champaran Satyagraha
- Haint blue
- Indigo revolt
- Red, White, and Black Make Blue
Images for kids