Lead(II) azide facts for kids
Quick facts for kids Lead(II) azide |
|
|---|---|
 |
|
 |
|
| IUPAC name | Diazidolead |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 236-542-1 |
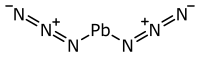
| SMILES | [N-]=[N+]=N[Pb]N=[N+]=[N-] |
|
InChI
InChI=1S/2N3.Pb/c2*1-3-2;/q2*-1;+2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | White powder |
| Density | 4.71 g/cm3 |
| Melting point | |
| 2.3 g/100 mL (18 °C) 9.0 g/100 mL (70 °C) |
|
| Solubility | Very soluble in acetic acid Insoluble in ammonia solution, NH4OH |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
462.3 kJ/mol |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
| Detonation velocity | 5180 m/s |
| Hazards | |
| Main hazards | Harmful, explosive |
| NFPA 704 |
|
| Related compounds | |
| Other cations | Potassium azide Sodium azide Copper(II) azide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Lead(II) azide (chemical formula: Pb(N
3)
2) is a type of inorganic compound. It is much more explosive than other similar chemicals called azides. Because it is so explosive, Pb(N
3)
2 is used in special devices called detonators. These detonators help to set off other, larger explosives. In its common form, it looks like a white or light brown powder.
How Lead Azide is Made
Lead(II) azide is made by mixing two other chemicals. These are sodium azide and lead(II) nitrate. This mixing happens in water. Sometimes, lead(II) acetate can be used instead of lead(II) nitrate.
To make the product safer and more stable, other substances are often added. These can be thickeners like dextrin or polyvinyl alcohol. These additions help to make the lead azide less sensitive. It is often sent out while still mixed in a dextrinated solution. This makes it safer to handle.
History of Production
A scientist named Theodor Curtius first made pure lead azide in 1891. However, it was very sensitive and hard to handle. So, in the 1920s and 1930s, a safer version was created. This version was mixed with dextrin (MIL-L-3055). The DuPont Company started making it in large amounts in 1932.
During World War II, there was a need for an even more powerful type of lead azide. This led to the development of RD-1333 lead azide (MIL-DTL-46225). This version used sodium carboxymethyl cellulose to help it form. Later, during the Vietnam War, a special type called Special Purpose Lead Azide (MIL-L-14758) was made. The US government also started storing large amounts of it.
After the Vietnam War, less lead azide was needed. By the early 1990s, the US stopped making it because they had so much stored. In the 2000s, people worried about how old the stored lead azide was. They started looking for ways to safely get rid of it and find new companies to make it.
Explosive Features
Lead azide is very sensitive. This means it can explode easily. Because of this, it is usually kept and handled under water. It is stored in special rubber containers that protect it.
It can explode if it falls about 150 millimeters (6 inches). It can also explode from a small spark of static electricity. Its explosion speed is very fast, around 5,180 meters per second (17,000 feet per second).
Small amounts of lead azide can be safely destroyed. This is done using chemicals like ammonium acetate and sodium dichromate.
Lead azide has a special quality called "immediate deflagration to detonation transition" (DDT). This means that even a small amount can fully explode. This can happen if it is touched by a flame or static electricity.
Lead azide can react with certain metals. These include copper, zinc, or cadmium. When it reacts, it can form other types of azides. For example, copper azide is even more explosive than lead azide. It is too sensitive to be used safely in products.
Lead azide was part of special bullets used in a shooting event on March 30, 1981. These bullets were designed to explode when they hit something. One of these bullets hit White House press secretary James Brady and likely exploded. Other bullets that hit people, including the one that hit President Ronald Reagan, did not explode.
See also
 In Spanish: Azida de plomo para niños
In Spanish: Azida de plomo para niños
- Lead styphnate
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |


