Nuclear reaction facts for kids
A nuclear reaction is a process where the nucleus (the center) of an atom changes. These reactions can create new atoms and release a lot of energy!
Contents
What happens in a nuclear reaction?
Imagine you're playing with LEGO bricks. A nuclear reaction is like taking apart a LEGO structure (the nucleus) and building something new. Sometimes, you might even get extra LEGOs (energy) in the process!
Atoms are made of tiny particles:
- Protons: These have a positive charge.
- Neutrons: These have no charge (they're neutral).
- Electrons: These have a negative charge and orbit around the nucleus.
The nucleus is where the protons and neutrons hang out. Nuclear reactions involve changes in the number or arrangement of these particles within the nucleus.
Types of nuclear reactions
There are a few main types of nuclear reactions:
Nuclear fission: splitting atoms
What it is: Nuclear fission is when the nucleus of an atom splits into two or more smaller nuclei. It's like taking a big LEGO castle and breaking it into smaller towers.
How it works: This usually happens when a neutron (a tiny particle) hits the nucleus of a heavy atom like uranium. The nucleus becomes unstable and splits, releasing energy and more neutrons.
Example: Nuclear power plants use nuclear fission to make electricity. They split uranium atoms, which releases heat. This heat boils water, creating steam that turns turbines to generate electricity.
Chain Reaction: The neutrons released during fission can hit other uranium atoms, causing them to split too. This is called a chain reaction. If it's not controlled, it can release a lot of energy very quickly, like in a nuclear explosion. In a nuclear reactor, special control rods are used to slow down the chain reaction and keep it at a safe level.
Fun Fact: Did you know that just a little bit of uranium, about the size of a golf ball, can create as much energy as burning a whole truckload of coal? That's how powerful nuclear fission is!
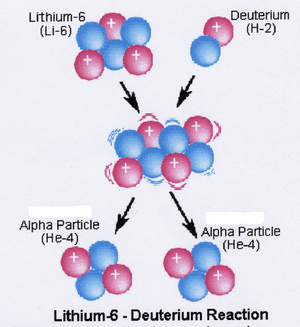
Nuclear fusion: joining atoms
What it is: Nuclear fusion is when two or more small nuclei join together to form a single, heavier nucleus. It's like taking two small LEGO cars and combining them to make a bigger, stronger car.
How it works: This happens when atoms are squeezed together with a lot of force.
Example: The sun and other stars use nuclear fusion to make energy. Deep inside the sun, hydrogen atoms are squeezed together so tightly that they fuse to form helium. This releases a huge amount of energy in the form of heat and light.
Why it's tricky: Scientists are trying to create nuclear fusion on Earth to generate electricity, but it's very difficult. You need extremely high temperatures and pressures to make atoms fuse together.
Fun Fact: Nuclear fusion is what makes the sun shine! Every second, the sun fuses about 600 million tons of hydrogen into helium, converting about 4 million tons of mass into energy.
Radioactive decay: atoms changing on their own
What it is: Radioactive decay is when an unstable nucleus spontaneously changes into a different nucleus by emitting particles or energy. It's like a LEGO structure that falls apart on its own because it wasn't built very well.
How it works: Some atoms are naturally unstable, meaning their nuclei have too many or too few neutrons. To become more stable, they release particles like alpha particles (two protons and two neutrons) or beta particles (electrons).
Example: Carbon-14 dating is used to find the age of old objects. Carbon-14 is a radioactive form of carbon that decays over time. By measuring how much carbon-14 is left in an object, scientists can figure out how old it is.
Fun Fact: Radioactive decay is used in medicine to treat certain diseases. Doctors use radioactive isotopes to target and destroy cancer cells.
History
The story of nuclear reactions is full of brilliant scientists making amazing discoveries!
- Henri Becquerel (1896): He accidentally discovered radioactivity while studying uranium. He noticed that uranium could expose photographic plates even in the dark, which meant it was giving off some kind of energy.
- Marie and Pierre Curie: They took Becquerel's discovery further and discovered other radioactive elements like polonium and radium. Marie Curie is famous for her work on radioactivity and was the first woman to win a Nobel Prize!
- Ernest Rutherford: He figured out that there were different types of radiation, which he called alpha, beta, and gamma rays. He also discovered that atoms have a nucleus.
- Otto Hahn and Fritz Strassmann (1938): These scientists discovered nuclear fission when they bombarded uranium with neutrons and found that it split into smaller atoms.
- Albert Einstein: His famous equation, E=mc², showed that mass and energy are related and that a small amount of mass can be converted into a huge amount of energy. This equation helps explain how nuclear reactions release so much energy.
Uses
Nuclear reactions have many uses in our world today:
- Generating electricity: Nuclear power plants use nuclear fission to produce electricity. They provide a large amount of energy without releasing greenhouse gases into the atmosphere.
- Medicine: Radioactive isotopes are used in medical imaging to see inside the body and diagnose diseases. They are also used in radiation therapy to treat cancer.
- Industry: Nuclear reactions are used in various industries, such as to sterilize medical equipment, preserve food, and measure the thickness of materials.
- Dating old objects: Carbon-14 dating is used to determine the age of ancient artifacts, fossils, and rocks.
- Space exploration: Nuclear power can be used to power spacecraft on long missions.
Fun Facts about nuclear reactions
- The energy released in a nuclear reaction comes from the strong force that holds the nucleus together.
- Nuclear reactions can change one element into another element. For example, in nuclear fusion, hydrogen atoms are turned into helium atoms.
- The first artificial nuclear reaction was performed by Ernest Rutherford in 1917. He bombarded nitrogen atoms with alpha particles and turned them into oxygen atoms.
- Nuclear reactions occur naturally in space, in stars, and even in our atmosphere.
- Scientists are still working on developing new and safer ways to use nuclear reactions to generate energy and solve other problems.
- Nuclear reactions differ from chemical reactions in that they do not need a catalyst. Radioactive decay also cannot be stopped, sped up or slowed down.
See also
 In Spanish: Procesos nucleares para niños
In Spanish: Procesos nucleares para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |