Electron facts for kids
An electron is a tiny piece of matter that is found in every atom. It has a negative electric charge and is one of the smallest known particles. Its symbol is e−.
Electrons are so small that scientists believe they cannot be broken down into anything even smaller. They are called elementary particles. Electrons are always moving, sometimes almost as fast as the speed of light.
Electrons are involved in many important forces, like gravity, electromagnetism, and weak interactions. The electricity that powers your radio, motors, and many other things is actually made of many electrons moving through wires or other materials that conduct electricity.
History of Electrons
People have known about electricity for a very long time! The ancient Greeks noticed that if you rubbed amber with fur, it could pick up small objects. This was one of the first times humans saw electricity in action, along with lightning.
In 1600, an English scientist named William Gilbert used the word electricus to describe this special ability of amber. The words electric and electricity come from the Greek word for amber, ēlektron.
In the early 1700s, scientists like Francis Hauksbee and C. F. du Fay thought there were two kinds of electricity. But later, Benjamin Franklin suggested that it was all one type of electricity, just with different amounts of it. He called these "positive" and "negative" charges.
In 1894, an Irish physicist named George Johnstone Stoney came up with the name electron for these tiny units of electricity. The word electron combines "electric" with a Greek ending that means "the way something is done."
Discovering the Electron
Scientists learned more about electrons by studying special glass tubes called cathode ray tubes. In the 1870s, an English scientist named Sir William Crookes made a very good cathode ray tube. He saw glowing rays inside the tube that moved from one end to the other. When he used a magnet, he could bend these rays, which showed they had a negative charge. He thought these rays were a new kind of matter.
Later, in 1890, Arthur Schuster did more experiments and found that these rays were indeed negatively charged. He tried to measure how much charge they had compared to their mass, but his results seemed too big at the time.
Then, in 1896, a British physicist named J. J. Thomson did some very important experiments. He proved that cathode rays were made of tiny, unique particles, not waves or atoms. He called these particles "corpuscles." Thomson found that these "corpuscles" were much, much lighter than the lightest known atom (hydrogen). He also showed that these negatively charged particles were the same no matter what material they came from. This was a huge discovery! The name electron was then widely accepted for these particles.
In 1909, two American physicists, Robert Millikan and Harvey Fletcher, did a famous experiment called the oil-drop experiment. They used an electric field to balance tiny oil droplets that had an electric charge. This allowed them to measure the exact charge of a single electron very precisely.
Around 1911, Charles Wilson invented the cloud chamber. This device allowed scientists to actually see the paths of fast-moving charged particles, like electrons, as they moved through a special vapor. It was like taking a photograph of their tiny trails!
What is an Electron Like?
Electrons have the smallest possible electrical charge. This charge is exactly opposite to the charge of a proton. Because of this, electrons are attracted to the positively charged protons in the center of an atom (called the nucleus). This attraction is what holds atoms together.
An electron is incredibly light, about 1,836 times lighter than a proton.
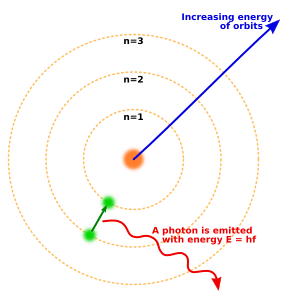
You can imagine electrons orbiting the nucleus of an atom, like planets around the sun. These orbits are called electron shells. Each shell is a different distance from the nucleus and can hold a certain number of electrons. The way electrons are arranged in these shells is called their electronic arrangement.
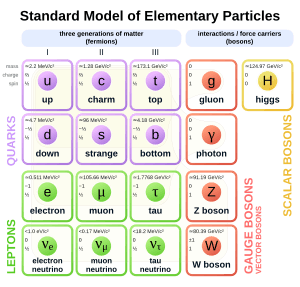
Electrons are part of a group of particles called leptons. Besides having a negative charge, electrons also have a property called spin. This spin makes them a type of particle called a fermion.
Most electrons are found inside atoms, but some can move freely in materials or in a vacuum. When electrons move together, it creates an electric current, which we call electricity.
If an object has more electrons than protons, it has a "negative charge." If it has fewer electrons than protons, it has a "positive charge." Electrons can move from one object to another, especially when objects touch or rub together. Objects with opposite charges attract each other, while objects with the same charge push each other away. When an object is "grounded," extra electrons move into the ground, making the object electrically neutral. This is how lightning rods work to protect buildings from lightning.
Chemical Reactions
The electrons in the outer shells of an atom are very important for chemical reactions. Atoms with full outer shells are usually not very reactive, meaning they don't easily combine with other atoms. But atoms with outer shells that are not full are very reactive. The number of electrons in an atom's outer shell helps explain how the periodic table of elements is organized.
Measuring Electrons
You can measure electric charge with a device called an electrometer. To measure electric current (the flow of electrons), you use a galvanometer.
'Seeing' an Electron
Scientists can't really "see" an electron with their eyes, but they have special tools called particle detectors that can observe how individual electrons behave. These tools can measure things like an electron's energy, spin, and charge. In one amazing experiment, scientists used a special trap called a Penning trap to hold a single electron for 10 months!
In 2008, scientists at Lund University in Sweden were able to capture the first video images of how an electron's energy is spread out. They used super-fast flashes of light, called attosecond pulses, to watch electrons move.
Anti-particles
Every particle has an "anti-particle." The anti-particle of an electron is called a positron. A positron is exactly like an electron but has a positive charge instead of a negative one. When an electron and a positron meet, they can destroy each other in a process called annihilation, releasing energy in the form of gamma rays.
How Electrons are Classified
In the world of particle physics, electrons belong to a group of particles called leptons. Leptons are thought to be elementary particles, meaning they are not made of smaller parts. Electrons are the lightest of all charged leptons. There are other charged leptons, like the muon and the tau, which are similar to electrons but much heavier. Leptons are different from quarks (which make up protons and neutrons) because they don't feel the strong interaction force. All leptons, including electrons, are fermions because they have a spin of 1/2.
Quantum Properties
Electrons are very strange! They can act like both a particle and a wave. This idea is called wave–particle duality. You can see this in the double-slit experiment, where a single electron can pass through two slits at the same time, like a wave, instead of just one, like a tiny ball.
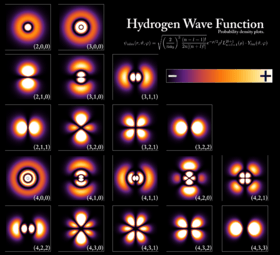
In quantum mechanics, the wave-like behavior of an electron is described by something called a wave function. If you square this function, it tells you the probability of finding the electron in a certain place.
Electrons are also considered identical particles. This means you can't tell one electron from another. In quantum mechanics, if you swap two electrons, the system's state doesn't change in a way you can observe. This leads to the Pauli exclusion principle, which says that no two electrons can be in the exact same quantum state. This principle is very important because it explains why electrons in an atom occupy different atomic orbitals instead of all piling up in the same one.
Electrons in Atoms and Molecules
An electron can be "bound" to the nucleus of an atom by the attractive electric force. An atom is a system of one or more electrons held around a nucleus. If an atom has a different number of electrons than protons, it's called an ion. The wave-like behavior of a bound electron is described by an atomic orbital. Each orbital has specific properties, like energy, and can hold up to two electrons, as long as they have opposite spins.
Electrons can move between different orbitals by absorbing or releasing tiny packets of light called photons. To escape an atom completely, an electron needs enough energy to overcome the force holding it to the nucleus. This happens in the photoelectric effect, for example, when light hits a material and knocks electrons out.
The way atoms form chemical bonds to create molecules is all thanks to electrons. The strongest bonds happen when atoms share or transfer electrons. Inside a molecule, electrons move around several nuclei and occupy molecular orbitals. A key idea here is electron pairs, where two electrons with opposite spins can share the same molecular orbital.
How Electrons Conduct Electricity

If an object has more or fewer electrons than protons, it has an overall electric charge. More electrons mean a negative charge, and fewer electrons mean a positive charge. If the number of electrons and protons is equal, the object is electrically neutral. You can give an object a charge by rubbing it, which is called the triboelectric effect.
Electrons that move freely, like in metals, are called "free electrons." When these free electrons move, they create an electric current, which also produces a magnetic field. This is how electricity works!
Different materials have different abilities to conduct electricity. Metals like copper and gold are good conductors because their electrons can move easily. Materials like glass and Teflon are poor conductors, called insulators, because their electrons are tightly held to their atoms. Semiconductors are in between, and their conductivity can be changed.
Metals are also good at conducting heat because their free electrons can carry thermal energy.
Motion and Energy
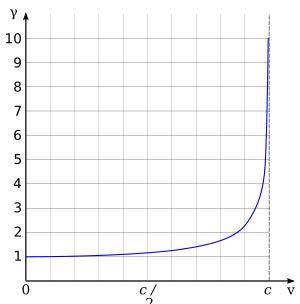
According to Einstein's theory of special relativity, as an electron moves faster and faster, its mass seems to increase from an observer's point of view. This makes it harder to speed it up even more. An electron can get very close to the speed of light in a vacuum, but it can never actually reach it. However, if electrons move very fast through a material like water, where light travels slower, they can actually go faster than light in that material! When this happens, they create a faint blue glow called Čerenkov radiation.
Because an electron acts like a wave, it has a specific wavelength depending on its speed. This wavelength is incredibly tiny, much smaller than an atom's nucleus, which allows scientists to use electrons to study very small structures.
Related pages
Images for kids
-
J. J. Thomson was a key scientist in discovering the electron.
-
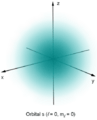
In quantum mechanics, the behavior of an electron in an atom is described by an orbital, which shows where you are most likely to find the electron. The shading shows the probability of finding the electron at different spots.
-
Pair production is when a photon (a particle of light) creates an electron and a positron (the electron's anti-particle) when it passes near an atomic nucleus.
-
Aurorae (like the Northern and Southern Lights) are mostly caused by energetic electrons hitting the Earth's atmosphere.
-
During a NASA wind tunnel test, a model of the Space Shuttle is hit by a beam of electrons to simulate what happens during re-entry into Earth's atmosphere.
See also
 In Spanish: Electrón para niños
In Spanish: Electrón para niños