Isotopes of plutonium facts for kids
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plutonium is a special element, mostly made by people. It's not found naturally, except for tiny amounts created when uranium atoms capture tiny particles called neutrons. Because it's mostly man-made, we don't have a "standard weight" for it like other elements.
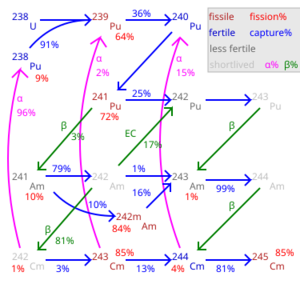
Plutonium has many different forms called isotopes. Think of isotopes like different versions of the same car model – they're all plutonium, but they have slightly different weights. All plutonium isotopes are radioactive, meaning they are unstable and slowly change into other elements over time. The first plutonium isotope, plutonium-238, was made in 1940. Scientists have found 20 different plutonium isotopes.
The most stable plutonium isotopes are:
- Plutonium-244, which takes about 80.8 million years to decay by half (its half-life).
- Plutonium-242, with a half-life of 373,300 years.
- Plutonium-239, with a half-life of 24,110 years.
- Plutonium-240, with a half-life of 6,560 years.
Plutonium isotopes have atomic weights ranging from 228 to 247. Lighter plutonium isotopes usually change into uranium or neptunium by breaking down (this is called alpha decay or spontaneous fission). Heavier isotopes usually change into americium by releasing electrons (this is called beta emission).
Contents
Understanding Plutonium Isotopes
Plutonium isotopes are different versions of the plutonium element. They all have 94 protons, but they have different numbers of neutrons. This difference in neutrons gives them different atomic weights and affects how they behave.
How Isotopes Change Over Time
Radioactive isotopes are unstable. They undergo "decay," which means they change into other elements by releasing energy and particles.
- Alpha decay: An atom releases an "alpha particle," which is like a tiny helium atom. This makes the original atom lighter.
- Beta decay: An atom releases an electron (a "beta particle"). This changes a neutron into a proton, making the atom a different element.
- Spontaneous fission: The atom's nucleus splits into two or more smaller nuclei, releasing a lot of energy and neutrons.
Important Plutonium Isotopes
Some plutonium isotopes are more important than others because of how they are used or how long they last.
Plutonium-238: A Space Powerhouse
Plutonium-238 has a half-life of about 87.7 years. This means half of it will decay in that time. It gives off alpha particles, which produce heat. This heat is used in special generators called radioisotope thermoelectric generators (RTGs) to power some spacecraft. It's made by capturing neutrons with neptunium-237.
Plutonium-239: Fuel for Energy and Weapons
Plutonium-239 has a half-life of 24,100 years. It is a "fissile" material. This means its nucleus can easily split apart when hit by slow neutrons. When it splits, it releases a lot of energy, gamma radiation, and more neutrons. These new neutrons can then hit other plutonium-239 atoms, causing a "nuclear chain reaction." This chain reaction is what powers nuclear reactors and is used in nuclear weapons. Plutonium-239 is made in nuclear reactors by hitting uranium-238 with neutrons.
Plutonium-240: The Bomb Blocker
Plutonium-240 has a high rate of spontaneous fission. This means it splits on its own more often than other isotopes. This spontaneous splitting releases neutrons, which can cause a chain reaction to start too early in a nuclear bomb. This makes it harder to build a powerful bomb with plutonium that has a lot of plutonium-240. Plutonium is often graded by how much plutonium-240 it contains:
- Weapons-grade: Less than 7% plutonium-240.
- Fuel-grade: 7–19% plutonium-240.
- Reactor-grade: More than 19% plutonium-240.
Lower grades are less suitable for bombs but can still be used in certain types of nuclear reactors.
Plutonium-241: A Shorter-Lived Fuel
Plutonium-241 is also fissile, meaning it can be split by neutrons. However, it has a shorter half-life of about 14 years. It mostly changes into americium-241 through beta decay. In a nuclear reactor, plutonium-241 is likely to split or capture another neutron before it decays.
Plutonium-242: Hard to Use
Plutonium-242 is not fissile, and it's hard to make it fissile. It also doesn't capture neutrons easily. It has a much longer half-life than lighter isotopes (about 375,000 years). This means it's not very radioactive and doesn't contribute much to the radioactivity of nuclear waste. However, it's not good for recycling in most nuclear reactors.
Plutonium-244: The Longest Lasting
Plutonium-244 is the most stable plutonium isotope, with a half-life of about 80 million years. It's not usually made in large amounts in nuclear reactors. Scientists have even found plutonium-244 in space!
Making and Using Plutonium
Plutonium isotopes are created in nuclear reactors and have important uses, especially in energy production and space exploration.
Making Plutonium-239
Plutonium-239 is the most common nuclear fuel after uranium-235. It's also the main fuel for the splitting part of nuclear weapons. It's made from uranium-238 in nuclear reactors. Here's how it happens: 1. Uranium-238 captures a neutron. 2. It then undergoes two beta decays, changing into plutonium-239.
Making Other Plutonium Isotopes
Other plutonium isotopes like plutonium-240, -241, and -242 are made when plutonium-239 and other isotopes capture more neutrons.
- Plutonium-239 and plutonium-241 are "fissile." This means they have a high chance of splitting when they capture a neutron.
- Plutonium-240 and plutonium-242 are not fissile with slow neutrons. They tend to build up in nuclear fuel that's been used for a long time in reactors.
Plutonium-241's Decay
Plutonium-241 has a half-life of 14 years. While it's in a reactor, it's more likely to split or capture a neutron than to decay. But in spent nuclear fuel that sits for years, much of the plutonium-241 will decay into americium-241. Americium-241 is a strong alpha emitter and is harder to use in reactors.
Plutonium-242's Challenges
Plutonium-242 doesn't easily capture neutrons. It takes many neutron captures for it to become a fissile isotope. This makes it less useful for recycling in most nuclear reactors. However, because its half-life is about 15 times longer than plutonium-239's, it's less radioactive and contributes less to the overall radioactivity of nuclear waste.
Making Plutonium-238 for Space
Plutonium-238 is not usually made in large amounts in typical nuclear fuel. However, it can be specially produced for RTGs. One way is to irradiate neptunium-237 with neutrons. This creates neptunium-238, which then decays into plutonium-238.
Plutonium-240 and Nuclear Weapons
Plutonium-240 is important because it affects how nuclear weapons are designed.
The Challenge of Plutonium-240
Plutonium-240 splits on its own (spontaneous fission) at a small but important rate. If there's too much plutonium-240 in the plutonium used for a nuclear bomb, the neutrons it releases can start the chain reaction too early. This causes the bomb to release its energy before it's fully ready, which greatly reduces its power.
To make a very powerful nuclear weapon, plutonium needs to be "weapons-grade," meaning it has more than 90% plutonium-239. Plutonium from commercial power reactors usually has at least 20% plutonium-240, making it "reactor-grade."
How Bombs Deal with Plutonium-240
Because of plutonium-240, nuclear weapons using plutonium must use a special design called the "implosion method." This method crushes the plutonium core very quickly to start the chain reaction. This is much harder to do than a simpler "gun-type" bomb design.
The difficulty of dealing with plutonium-240 has actually been a good thing for preventing the spread of nuclear weapons. Implosion bombs are complex to build, which makes it harder for more countries to create them. Implosion bombs are also safer and less likely to explode by accident than gun-type bombs.
Images for kids
-
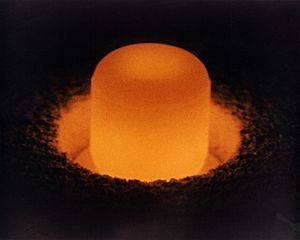
A pellet of 238Pu, glowing from its own heat, used for radioisotope thermoelectric generators.
 | Anna J. Cooper |
 | Mary McLeod Bethune |
 | Lillie Mae Bradford |