Metallic bonding facts for kids
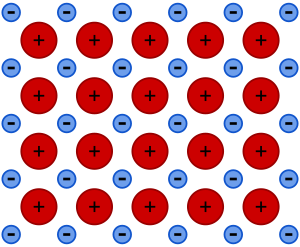
Metallic bonding is a special way that metal atoms stick together. Imagine metal atoms as positive ions floating in a "sea" of shared, free-moving electrons. These electrons are not tied to just one atom; they move around all the atoms in the metal. This strong connection between the positive metal ions and the shared negative electrons is what we call metallic bonding.
This unique type of bonding helps explain why metals have many of their cool properties. For example, it makes metals strong, easy to bend or shape (like ductility and malleability), good at carrying heat and electricity, and why they look shiny (luster).
Sometimes, metals can also have other types of bonds, even when they are pure. For instance, the metal gallium has some covalent bonds (where atoms share electrons in a more fixed way) between pairs of atoms, along with metallic bonding.
Contents
How Metallic Bonding Works
Metallic bonding happens because electrons in metals are not stuck to one atom. They are "delocalized," meaning they can move freely throughout the whole metal structure. Also, there are many more places for these electrons to go than there are electrons, which helps them move easily.
In 2D and 3D
Think of graphene, which is a super-thin, two-dimensional material. It has metallic bonding similar to how carbon atoms bond in rings, like in benzene.
In three dimensions, like in a chunk of metal, this sharing of electrons is even more extreme. You can think of a whole piece of metal as one giant "molecule" where all the outer electrons are shared everywhere. This is why you can't usually tell individual molecules apart in a solid metal. Metallic bonding is usually not "polar," meaning electrons are shared pretty evenly, especially in pure metals. It's like a very spread-out form of covalent bonding.
For some metals, like caesium, the electrons are so free that they almost act like a gas trapped inside the metal. For other metals, the electrons are still influenced by the metal atoms, but they can still move around a lot.
Why Metals Conduct and Bend Easily
Metal atoms have only a few electrons in their outermost shells. Because these electrons are shared and can move freely, they can easily switch from one energy level to another. This is why metals are great at conducting electricity. When you apply an electric field, these free electrons simply start moving in one direction, creating an electric current.
The freedom of electrons also explains why metals are malleable (can be hammered into shapes) and ductile (can be pulled into wires). When you push or pull on a metal, the layers of atoms can slide past each other. The shared electrons quickly form new connections, so the metal doesn't break easily. However, if a metal has impurities (other elements mixed in), it can become harder because these impurities block the easy sliding of atoms. For example, pure gold is very soft, which is why it's often mixed with other metals for jewelry.
Metals are also good heat conductors. While the free electrons help, the main way heat travels through metals is through vibrations of the atoms themselves, called phonons.
Metallic Radius
The metallic radius is basically half the distance between two metal atoms that are next to each other in a metal structure. This size can change a bit depending on how many other atoms are touching it (called the coordination number), and also on the temperature and pressure.
Scientists sometimes adjust these radii to compare them better, imagining all atoms are surrounded by 12 other atoms. This adjustment helps them see trends in how atom sizes change across the periodic table. Generally, metal atoms get smaller as you move across a period (row) because the positive pull from the nucleus gets stronger. But they get bigger as you move down a group (column) because they have more electron shells.
Strength of Metallic Bonds
The forces holding metal atoms together are very strong. This is why metals often have high boiling points, meaning it takes a lot of energy to turn them into a gas. For example, tungsten has an extremely high boiling point.
However, some metals, like zinc, cadmium, and mercury, have lower boiling points. This is because their outer electrons are held a bit more tightly, making them less "free" compared to other metals.
Even when metals melt, their bonds can still be very strong. Gallium, for instance, melts easily in your hand, but it has a very high boiling point. This shows that the strong metallic bonding is still present even in liquid form.
The strength of metallic bonds doesn't depend much on direction. This is because the electrons are shared everywhere, not just between two specific atoms. This lack of direction helps metals form simple, tightly packed structures.
Metals can even form glasses if they are cooled very quickly. These glasses have a messy, unorganized structure but still have metallic bonding.
Solubility and Metal Mixtures
Metals usually don't dissolve in water or other liquids unless they react with them and lose their metallic bonding. However, metals often dissolve easily in each other, forming alloys, and they keep their metallic properties. For example, gold dissolves well in mercury even at room temperature.
Sometimes, two metals can mix completely to form a solid solution, like electrum, which is an alloy of silver and gold. Other times, metals can form specific compounds with different structures, often called intermetallic compounds. Because metallic bonding is so spread out, these compounds don't always follow simple rules about how much of each metal is present.
Optical Properties of Metals
The "sea" of mobile electrons in metals greatly affects how they interact with light.
When light hits a metal, the electric part of the light wave makes the free electrons move. This causes most of the light to be reflected, which is why metals look shiny and often silvery or grayish. The balance between how much light is reflected and how much is absorbed determines if a metal looks more white or more gray. Silver, being a great conductor, is one of the whitest metals.
Some metals, like reddish copper and yellowish gold, have colors. This happens because there's a limit to how fast the metallic electrons can respond to light. For copper and gold, this limit is closer to the visible light spectrum. They reflect most colors but absorb some, giving them their unique hues.
At the surface of a metal, the free electrons can also create special waves called "surface plasmons." These are like ripples in the electron sea. When light hits tiny metal particles, like in colloidal gold, these plasmons can absorb light very strongly, creating intense colors like purple-red.
See also
 In Spanish: Enlace metálico para niños
In Spanish: Enlace metálico para niños
- Atomic radii of the elements (data page)
- Bonding in solids
- Metal aromaticity