Naphthalene facts for kids
Quick facts for kids Naphthalene |
|
|---|---|
 |
|
 |
|
| Other names | white tar, camphor tar, tar camphor, naphthalin, naphthaline, antimite, albocarbon, hexalene, mothballs, moth flakes |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:16482 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | White solid crystals/ flakes |
| Odor | Strong smell, like coal tar |
| Density | 1.145 g/cm3 (at 15.5 °C) |
| Melting point | |
| Boiling point | |
| 31.6 mg/L (at 25 °C) in water | |
| Solubility | Dissolves well in alcohols, benzene, toluene, and other similar liquids. |
| Vapor pressure | 8.64 Pa (at 20 °C) |
| Structure | |
| Crystal structure | Monoclinic (a type of crystal shape) |
| Hazards | |
| Main hazards | Can easily catch fire, may cause allergic reactions, and might cause cancer. Its dust can explode when mixed with air. |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Naphthalene is a chemical that looks like white, solid crystals or flakes. It has a very strong smell, often described as smelling like coal tar. You might know it best as the main ingredient in mothballs, which are used to protect clothes from insects. It's also found in some urinal deodorizer blocks and can be used as an antiseptic to kill germs.
In mothballs, naphthalene works as an insecticide or pesticide, meaning it kills insects and other pests.
It's important to know that naphthalene is toxic, which means it can be harmful. If people are exposed to too much naphthalene, it can damage their red blood cells. Scientists are also studying if naphthalene can cause cancer.
The Story of Naphthalene
Naphthalene was first discovered in the early 1820s. Two different groups of scientists wrote about a substance that matched naphthalene's description. They both made it by heating and collecting vapors from coal tar.
In 1821, a scientist named John Kidd brought together what both groups had found. He accurately described what naphthalene was like and how to make it. Kidd named it "naphthalene" because the word "naphtha" was used for any explosive mixture made from hydrocarbons.
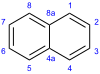
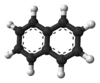
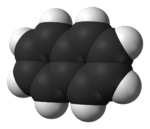
A few years later, in 1826, Michael Faraday figured out the exact chemical formula for naphthalene. Then, in 1866, Emil Erlenmeyer suggested that naphthalene was made of two benzene rings joined together. Three years later, in 1869, Carl Gräbe proved that this idea was correct.
Images for kids
See also
 In Spanish: Naftalina para niños
In Spanish: Naftalina para niños