Dinitrogen tetroxide facts for kids
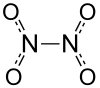
Dinitrogen tetroxide, also known as nitrogen tetroxide, is a special chemical compound. Its chemical formula is N2O4. It's made of nitrogen and oxygen atoms. This compound is very important in chemistry and even in space travel!
Quick facts for kids Dinitrogen tetroxide |
|
|---|---|
 |
|
| IUPAC name | Dinitrogen tetraoxide |
| Other names | Dinitrogen(II) oxide(-I) |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 234-126-4 |
| ChEBI | CHEBI:29803 |
| RTECS number | QW9800000 |
| SMILES | [O-] [N+](=O)[N+]([O-])=O |
|
InChI
InChI=1/N2O4/c3-1(4)2(5)6
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colourless liquid / orange gas |
| Density | 1.44246 g/cm3 (liquid, 21 °C) |
| Melting point | |
| Boiling point | |
| reacts | |
| Vapor pressure | 96 kPa (20 °C) |
| Refractive index (nD) | 1.00112 |
| Structure | |
| Molecular shape | planar, D2h |
| Dipole moment | zero |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
+9.16 kJ/mol |
| Standard molar entropy S |
304.29 J K−1 mol−1 |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Very toxic (T+) Corrosive (C) |
| EU Index | 007-002-00-0 |
| NFPA 704 |
|
| R-phrases | R26, R34 |
| S-phrases | (S1/2), S9, S26, S28, S36/37/39, S45 |
| Flash point | Non-flammable |
| Related compounds | |
| Related nitrogen oxides | Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen pentoxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
What it Looks Like and Does
Dinitrogen tetroxide is usually a clear, colorless liquid. But it can also be an orange-brown gas. This happens because it often mixes with another gas called nitrogen dioxide.
It's a very strong chemical. It can eat away at other materials, which means it's very corrosive. It's also a powerful oxidizing agent. This means it can cause other substances to lose electrons, which often leads to strong reactions. For example, it can even catch fire if it touches a chemical called hydrazine.
How it's Made
This chemical is made by joining two molecules of nitrogen dioxide together. This process usually happens when the temperature is very low or the pressure is very high.
Cool Uses
Dinitrogen tetroxide has some exciting uses!
- Rocket Fuel: It's used as a special fuel part called a propellant for rockets. It's often paired with hydrazine. What's cool about this mix is that it doesn't need a spark or flame to ignite. The two chemicals react on their own when they touch, which is very useful for rockets.
- Making Acids: It's also used to help make nitric acid. Nitric acid is a strong acid used in many industries.
- Reacting with Metals: Dinitrogen tetroxide can react with different metals. When it does, it forms new compounds called nitrates.
Safety First
Dinitrogen tetroxide is a very strong chemical and can be dangerous. It is highly toxic, meaning it can be harmful if you breathe it in or get it on your skin. It's also very corrosive, so it can cause serious burns.
Some astronauts have accidentally breathed in small amounts of this chemical. When this happened, they needed to go to the hospital for medical care. This shows how important it is to handle such chemicals with extreme caution and proper safety gear.
Related pages
See also
 In Spanish: Tetróxido de dinitrógeno para niños
In Spanish: Tetróxido de dinitrógeno para niños