Geiger–Marsden experiments facts for kids
The Geiger–Marsden experiments, also known as the Rutherford gold foil experiment, were a very important series of experiments that helped scientists understand what an atom is really like. These experiments showed that every atom has a tiny, dense center called a nucleus. This nucleus holds almost all of the atom's weight and all of its positive charge. Scientists figured this out by watching how tiny particles, called alpha particles, bounced off a thin piece of metal foil. The experiments happened between 1908 and 1913. They were done by Hans Geiger and Ernest Marsden, guided by Ernest Rutherford, at the University of Manchester.
Contents
Understanding the Atom: Before Rutherford
The "Plum Pudding" Atom Model
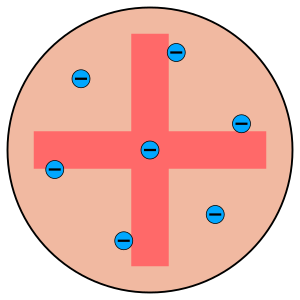
Before Rutherford's experiments, the most popular idea about atoms was called the "plum pudding model". This model was created by J. J. Thomson, who had discovered the tiny, negatively charged particles called electrons. Thomson thought that an atom was like a soft, positively charged ball, with the electrons scattered inside it, much like raisins in a Christmas pudding. At this time, no one knew about protons (positive particles) or neutrons (neutral particles) in the atom. This old model was based on simple physics, but today we use more advanced ideas called quantum mechanics to describe atoms.
Not everyone agreed with Thomson's model, even before Rutherford's experiments. For example, a Japanese scientist named Hantaro Nagaoka thought that positive and negative charges couldn't mix together like that. He suggested that electrons might orbit the positive charge, similar to how the rings orbit Saturn.
What are Alpha Particles?
An alpha particle is a very small, positively charged piece of matter. It's naturally released by some radioactive elements. Rutherford found these particles and realized they were like helium atoms but without their electrons. Again, remember that protons and neutrons hadn't been discovered yet!
What Thomson's Model Predicted
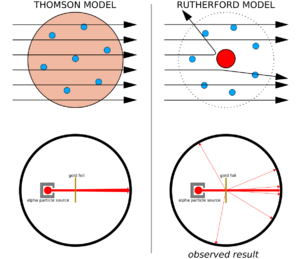
According to Thomson's "plum pudding" model, if an alpha particle hit an atom, it should just pass right through. Its path might bend only a tiny bit, maybe less than one degree. Think of it like throwing a tennis ball through a cloud – it wouldn't bounce back! This is because in Thomson's model, the positive and negative charges were spread out over the whole atom. Rutherford calculated that these spread-out electric forces would be too weak to push a fast, heavy alpha particle very much.
The Amazing Discovery: What Really Happened
Unexpected Results from the Experiments
Between 1908 and 1913, Rutherford, Geiger, and Marsden did many tests. They aimed a beam of alpha particles at very thin sheets of different metals. They watched where the alpha particles went using a special screen that glowed when hit.
What they saw was truly surprising! Some alpha particles were scattered in all directions, even bouncing back at angles greater than 90 degrees. This was impossible according to Thomson's model. It was like firing a cannonball at a piece of tissue paper and having it bounce back at you! These particles must have hit a much stronger electric force than anyone expected.
Rutherford's New Idea: The Atomic Nucleus
These strange results made Rutherford realize something big in 1911. He concluded that an atom is mostly empty space. But, it has a tiny, super-dense center where all its positive charge is packed. This tiny center is what we now call the nucleus.
How This Changed Science
Even other scientists were amazed. Hantaro Nagaoka, who had his own idea of the atom, wrote to Rutherford saying he was impressed by the simple setup and amazing results. The famous astronomer Arthur Eddington called Rutherford's discovery the most important scientific achievement since the idea of the atom was first thought of by Democritus ages ago.
Rutherford's model also showed that the old rules of physics (called Newtonian physics) didn't work for tiny things like atoms. For example, if electrons orbited the nucleus like planets, they should lose energy and spiral into the nucleus. But this wasn't happening! This meant that new rules were needed for the atomic world. This led to the development of quantum mechanics by scientists like Niels Bohr. Around the same time, Albert Einstein showed that old physics rules also didn't work for huge things like galaxies with his theory of general relativity.
Rutherford himself later talked about how shocked he was by the results. He said it was "quite the most incredible event that has ever happened to me in my life." He compared it to firing a large shell at tissue paper and having it bounce back. This amazing result made him realize that most of an atom's mass must be in a tiny, dense center.
The Experiments: How They Were Done
The Team Behind the Discovery
Ernest Rutherford was a physics professor at the University of Manchester. He was already famous for his work on radiation, having discovered alpha rays, beta rays, and gamma rays. He also proved that these rays came from atoms breaking down (called radioactive decay). In 1906, a German physicist named Hans Geiger visited Rutherford, who was so impressed that he asked Geiger to stay and help with his research. Ernest Marsden was a young student working with Geiger.
Alpha particles are tiny, positively charged particles given off by substances like uranium and radium. Rutherford had found them in 1899. He wanted to measure their exact charge and mass. To do this, he needed to count how many alpha particles his radium sample was releasing. Alpha particles are too small to see, but Rutherford knew they could make air molecules electrically charged (ionize them). If this charged air was in an electric field, it would create a small electric current.
Based on this idea, Rutherford and Geiger built a simple counting device. It had two metal parts (electrodes) in a glass tube. Every alpha particle that passed through the tube would create a tiny pulse of electricity that could be counted. This was an early version of the Geiger counter.
However, their first counter wasn't perfect. The alpha particles were bending too much when they hit air molecules inside the tube. This made the readings jump around. Rutherford was puzzled because he thought alpha particles were too heavy to be pushed around so easily. He asked Geiger to find out how much matter could scatter alpha rays.
The experiments they designed involved shooting alpha particles at thin metal foils. They wanted to see how the foil scattered the particles based on its thickness and what metal it was made of. They used a special screen that glowed when an alpha particle hit it. Each hit made a tiny flash of light. Geiger spent hours in a dark lab, counting these tiny flashes through a microscope. Rutherford, being older, didn't have the patience for this detailed work, so he left it to his younger helpers. They tried different metals for the foil, but they liked gold best because it could be made very, very thin. For the alpha particles, Rutherford chose radon, which was millions of times more radioactive than uranium.
The 1908 Experiment: Small Bends
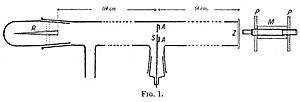
In 1908, Geiger wrote about an experiment he did. He used a long glass tube, almost two meters long. At one end, there was a source of alpha particles (R). At the other end, there was a glowing screen (Z). In the middle, there was a narrow slit. Alpha particles from the source went through the slit and made a bright spot on the screen. Geiger used a microscope (M) to count the flashes and see how spread out they were.
First, Geiger removed all the air from the tube. The alpha particles made a clear, sharp image on the screen, matching the slit's shape. Then, he let some air into the tube, and the glowing spot became blurry. Next, he removed the air again and put some gold foil over the slit (at AA). This also made the light spot spread out. This experiment showed that both air and solid matter could scatter alpha particles. However, this setup could only see small bending angles. Rutherford wanted to know if alpha particles could be scattered by much larger angles, maybe even more than 90 degrees.
The 1909 Experiment: Big Bounces!
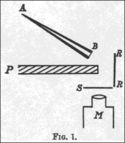
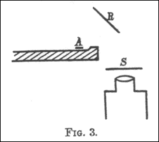
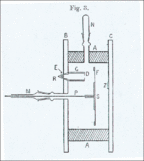
In 1909, Geiger and Marsden wrote about the experiment that proved alpha particles could indeed bounce back at angles greater than 90 degrees. They used a small glass tube (AB) with radioactive substances inside, which was their alpha particle source. They set up a lead plate (P) and, behind it, a glowing screen (S). The alpha particle source was placed so that its particles couldn't directly hit the screen. They still saw a few flashes on the screen because some alpha particles bounced off air molecules around the plate.
Then, they placed a metal foil (R) next to the lead plate. They aimed the alpha particle source at the foil to see if particles would bounce off it and hit the screen. They saw a clear increase in the number of flashes on the screen! They counted the flashes and found that heavier metals like gold reflected more alpha particles than lighter ones like aluminum.
Geiger and Marsden then wanted to figure out how many alpha particles were actually bouncing back. They used a different setup, placing a small amount of radium C on the lead plate, which bounced off a platinum reflector (R) and onto the screen. They found that only a tiny number of alpha particles that hit the reflector actually bounced onto the screen – only about 1 out of every 8,000!
The 1910 Experiment: Measuring the Bends
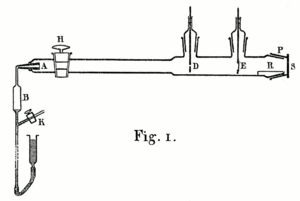
In 1910, Geiger described an experiment to measure exactly how the bending angle of alpha particles changed. He wanted to see how it varied with the material, its thickness, and the speed of the alpha particles. He built an airtight glass tube with all the air pumped out. At one end was a bulb (B) with radon. The radon was moved into a narrow glass pipe (A) blocked by a thin piece of mica. At the other end of the tube was a glowing zinc sulfide screen (S).
Geiger used a microscope to count the flashes on the screen. The microscope was attached to a special scale, allowing him to measure exactly where the flashes appeared. This helped him calculate the particles' bending angles. The alpha particles from A were narrowed into a beam by a small hole at D. Geiger placed metal foil in the path of the rays at D and E to see how the glowing area changed. He could also change the speed of the alpha particles by adding more thin sheets of mica or aluminum at A.
From his measurements, Geiger learned a few things:
- The most common angle of bending increased with the thickness of the material.
- The most common angle of bending was related to the atomic weight of the substance.
- The most common angle of bending decreased when the alpha particles moved faster.
- It was extremely rare for a particle to be bent by more than 90 degrees.
Rutherford's Math: Explaining the Scattering
After seeing these experiment results, Rutherford published a very important paper in 1911. In it, he suggested that the atom has a very small, very strong center of electric charge. For his math, he imagined this center was a single point of positive charge.
Rutherford then created a mathematical equation that showed how the foil should scatter the alpha particles if all the positive charge and most of the atom's weight were packed into a tiny point at the center of the atom. This equation helped explain the surprising results.
The 1913 Experiment: Proving the Math
In 1913, Geiger and Marsden wrote another paper describing experiments to check if Rutherford's equation was correct. Rutherford's equation predicted that the number of flashes seen at a certain angle should depend on:
- The angle of deflection (how much it bends).
- The thickness of the foil.
- The strength of the central charge (the nucleus).
- The speed of the alpha particles.
Their 1913 paper described four experiments that proved each of these predictions.
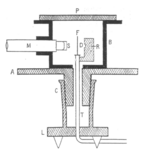
To test how scattering changed with the angle, Geiger and Marsden built a special device. It was a hollow metal cylinder that could spin. Inside, there was a metal foil (F) and a source of radon (R). A microscope (M) with a glowing screen (S) poked through the cylinder's wall and pointed at the foil. By spinning the cylinder, the microscope could move all the way around the foil. This allowed Geiger to count alpha particles that bounced back at angles up to 150 degrees. After accounting for small errors, Geiger and Marsden found that the number of alpha particles scattered at a certain angle matched Rutherford's prediction.
Next, Geiger and Marsden tested how scattering changed with the foil's thickness. They used a disc (S) with six holes. These holes were covered with metal foils (F) of different thicknesses, or left open for comparison. This disc was sealed inside a brass ring (A) between two glass plates (B and C). They could spin the disc to bring each foil in front of the alpha particle source (R). On the back glass plate was a zinc sulfide screen (Z). Geiger and Marsden found that the number of flashes on the screen was indeed related to the thickness, as long as the foil was thin.
Using the same setup, they also tested how scattering changed with the square of the nucleus's charge. Geiger and Marsden didn't know the exact positive charge of the nucleus for their metals (they had only just discovered the nucleus!). But they assumed it was related to the atomic weight. So, they tested if the scattering was related to the atomic weight squared. They used foils of gold, tin, silver, copper, and aluminum. They measured how many flashes each foil produced. They found that the results matched Rutherford's prediction: scattering was related to the square of the atomic weight, which meant it was related to the square of the nucleus's charge.
Finally, Geiger and Marsden tested how scattering changed with the speed of the alpha particles. Using the same equipment, they slowed down the alpha particles by putting extra sheets of mica in front of the source. They found that the number of flashes was indeed related to the inverse of the alpha particle's speed raised to the fourth power, just as Rutherford predicted.
Rutherford Confirms the Nucleus is Positive
In his 1911 paper, Rutherford had guessed that the atom's central charge was positive. But, a negative charge would have also fit his scattering model. In a 1913 paper, Rutherford declared that the "nucleus" (as he now called it) was definitely positively charged. He based this on later experiments where alpha particles were scattered in different gases.
In 1917, Rutherford and his assistant William Kay started looking at how alpha particles moved through gases like hydrogen and nitrogen. In one experiment, they shot alpha particles through hydrogen. The alpha particles pushed the hydrogen nuclei forward, not backward. In another experiment, when they shot alpha particles through nitrogen, they found that the alpha particles actually knocked hydrogen nuclei (which are protons) out of the nitrogen nuclei. This proved the nucleus was positive.
See also
 In Spanish: Experimento de Rutherford para niños
In Spanish: Experimento de Rutherford para niños
- Atomic theory
- Rutherford backscattering spectroscopy
- Rutherford scattering
- List of scattering experiments