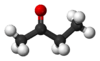Butanone facts for kids
Quick facts for kids Butanone |
|
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
Butan-2-one
|
|
| Other names |
|
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C02845 |
| ChEBI | CHEBI:28398 |
| RTECS number | EL6475000 |
| SMILES | O=C(C)CC |
|
InChI
InChI=1/C4H8O/c1-3-4(2)5/h3H2,1-2H3
|
|
| Beilstein Reference | 741880 |
| Gmelin Reference | 25656 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless liquid |
| Odor | Mint or acetone-like |
| Density | 0.8050 g/mL |
| Melting point | |
| Boiling point | |
| 27.5 g/100 mL | |
| log P | 0.37 |
| Vapor pressure | 78 mmHg (20 °C) |
| Acidity (pKa) | 14.7 |
| −45.58·10−6 cm3/mol | |
| Refractive index (nD) | 1.37880 |
| Viscosity | 0.43 cP |
| Structure | |
| Dipole moment | 2.76 D |
| Hazards | |
| EU classification | Flammable (F) Irritant (Xi) |
| NFPA 704 |
|
| R-phrases | R11 R36 R66 R67 |
| S-phrases | (S2) S9 S16 |
| Explosive limits | 1.4–11.4% |
| U.S. Permissible exposure limit (PEL) |
TWA 200 ppm (590 mg/m3) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
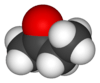
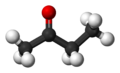
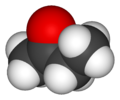
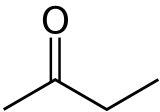
Butanone, also known as methyl ethyl ketone (MEK), is a common organic compound. It has the chemical formula C4H8O. This means it is made of four carbon atoms, eight hydrogen atoms, and one oxygen atom. It is a simple type of chemical called a ketone.
Butanone is a clear liquid. It has a strong, sweet smell, a bit like butterscotch or acetone. It can mix well with water. Because of this, it is often used as a solvent, which means it can dissolve other substances.
Contents
What is Butanone?
Butanone is a chemical compound. It belongs to a group of chemicals called ketones. Ketones are organic compounds that have a special group of atoms called a carbonyl group. This group has a carbon atom double-bonded to an oxygen atom.
How is Butanone structured?
Butanone has a chain of four carbon atoms. One of the middle carbon atoms is double-bonded to an oxygen atom. This specific arrangement gives butanone its unique properties. Its chemical formula, C4H8O, tells us exactly how many atoms of each element it contains.
What are the properties of Butanone?
Butanone has several interesting properties. These properties make it useful for many different jobs.
- Appearance: It is a clear, colorless liquid.
- Smell: It smells like mint or acetone. Some people describe it as sweet, like butterscotch.
- Density: It is lighter than water, with a density of about 0.805 grams per milliliter.
- Solubility: It can dissolve in water. About 27.5 grams of butanone can dissolve in 100 milliliters of water.
- Melting and Boiling Points: Butanone freezes at a very cold temperature, around -86 degrees Celsius. It boils at about 79.64 degrees Celsius. This means it turns into a gas at a temperature slightly higher than room temperature.
How is Butanone used?
Butanone is a very useful chemical. Its ability to dissolve many things makes it popular in different industries.
- Solvent: Its main use is as a solvent. It can dissolve many plastics, resins, and glues. This makes it great for things like:
- Removing paint and varnish.
- Cleaning surfaces.
- Making glues and adhesives.
- Manufacturing: It is used in making plastics, textiles, and even some types of wax.
- Cleaning Agent: Because it dissolves grease and oils, it's used to clean metal surfaces and electronic parts.
Is Butanone safe to use?
Like many chemicals, butanone needs to be handled carefully. It is important to know about its safety features.
- Flammable: Butanone is very flammable. This means it can easily catch fire. It should always be kept away from sparks, flames, and heat.
- Irritant: It can irritate your eyes, skin, and respiratory system if you breathe in too much.
- Ventilation: When using butanone, it's important to have good air circulation. This helps to prevent too much vapor from building up in the air.
Always follow safety instructions when working with chemicals.
Images for kids
See also
 In Spanish: Butanona para niños
In Spanish: Butanona para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |