Desalination facts for kids
Desalination is a special process that takes out extra salt and other minerals from water. This makes the water fresh and safe for animals to drink or for irrigation (watering crops).
Sometimes, when almost all the salt is removed for people to drink, the process can also create table salt as a side product.
Many countries use desalination. For example, the United States uses it to treat water for Mexico. Countries in the Middle East, like Saudi Arabia and the United Arab Emirates, use a lot of desalinated water for agriculture because they have plenty of energy. Saudi Arabia's plants make about 24% of the world's desalinated water. The Jebel Ali plant in the UAE is one of the biggest, making 300 million cubic meters of water each year.
Today, there are about 21,000 desalination plants worldwide. The largest ones are in the United Arab Emirates, Saudi Arabia, and Israel. The biggest plant in the world is the Ras Al-Khair Power and Desalination Plant in Saudi Arabia, which can produce 1,401,000 cubic meters of water per day.
Contents
History of Desalination
People have known about desalination for thousands of years. The ancient Greek thinker Aristotle noticed that "salt water, when it turns into vapour, becomes sweet." This means he saw that when salty water evaporates, the steam is fresh, and it doesn't turn salty again when it cools down. For a long time, desalination was only done on a small scale.
One of the first big land-based desalination plants might have been built in 1560 on an island near Tunisia. Spanish soldiers were trapped there and needed fresh water. Their captain supposedly built a machine that could make 40 barrels of fresh water every day.
Before the Industrial Revolution, desalination was mostly important for ships at sea. Ships needed to carry large amounts of fresh water for their journeys.
Around 1800, things changed quickly with the invention of the steam engine. People learned more about how steam works, and there was a need for pure water for steam boilers. This helped improve water distilling systems. Also, as European countries expanded their influence around the world, there was a greater need for fresh water in distant places.
After World War II, the United States did a lot of research on better desalination methods. The first industrial desalination plant in the US opened in Freeport, Texas, in 1961. It was hoped to help the region after a long drought. President John F. Kennedy even called desalination "a work that in many ways is more important than any other scientific enterprise."
The first commercial plant using a method called reverse osmosis opened in California in 1965. It treated slightly salty water. A few years later, in 1975, the first reverse osmosis plant for sea water started working.
Why Desalination is Used
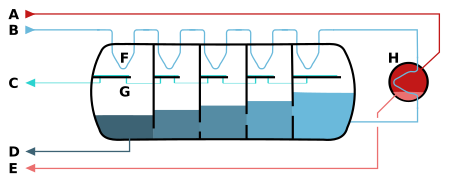
A – steam in B – seawater in C – clean water out
D – salty water out (waste) E – condensed water out F – heat exchange G – collected clean water
H – heater
This machine acts like a heat exchanger. A vacuum pump lowers the pressure inside, which helps the heated seawater turn into steam. The steam then turns back into water on pipes where fresh sea water is flowing.
Desalination can help solve water shortages in areas that don't have enough fresh water. However, for places far from the sea, like New Delhi, or in high places, like Mexico City, it might be cheaper to bring water from somewhere else. Desalinated water can also be expensive, so poorer regions might not be able to use this technology. But for cities near the coast, desalination is becoming a good option.
In 2023, Israel started using desalinated water to refill the Sea of Galilee, which is a large freshwater lake.
Environmental Concerns
Desalination plants can affect the environment.
Water Intake
When plants take in water from the ocean, they can suck in fish, shellfish, or their eggs. These tiny creatures can be harmed or killed by the heat, chemicals, or physical stress inside the plant. Larger animals can get trapped against screens at the water intake. Some newer plants use special wells on the beach to take in water, which helps protect marine life, but these can cost more energy and money.
For example, the Kwinana Desalination Plant in Perth, Australia, opened in 2007. It takes in water very slowly, at about 0.1 meters per second. This speed allows fish to swim away and avoid being sucked in. This plant provides about 140,000 cubic meters of clean water every day.
Salty Outflow
Desalination processes create a lot of very salty water called brine. This brine can be warmer than the ocean water and might contain leftover chemicals from the treatment process.
Brine is heavier than regular seawater, so it sinks to the bottom of the ocean. This can harm the ocean ecosystem. Studies have shown that brine can spread several kilometers from the plant, potentially affecting marine life far away. Careful planning and environmental studies can help reduce this problem.
Health Aspects
Desalination removes iodine from water. If people drink only desalinated water without getting iodine from other sources, it could increase their risk of iodine deficiency problems.
Methods of Desalination
There are several ways to desalinate water. Each method has its good points and bad points.
Vacuum Distillation
This is a traditional method. It involves boiling water to leave the impurities (like salt) behind. In desalination, the air pressure is lowered, which means the water boils at a much lower temperature. This allows the plant to use "waste" heat from other processes, like making electricity.
Multi-Stage Flash Distillation
In this method, water is evaporated and separated from seawater through a series of steps called "flash evaporation." Each step uses the heat released from the steam of the previous step. It's like a chain reaction where heat is reused.
Multiple-Effect Distillation
This method works through a series of steps called "effects." Incoming water is sprayed onto heated pipes, which creates steam. This steam is then used to heat the next batch of seawater. To save energy, the steam can come from nearby power plants.
Vapor-Compression Distillation
This method uses a machine or a jet stream to compress the steam above the liquid. This compressed steam then provides the heat needed to evaporate the rest of the seawater. This system mainly needs electricity and works best for smaller plants.
Reverse Osmosis
This is a very common method. It uses special membranes to remove salt from water. Pressure is applied to the salty water, pushing the pure water through the membrane while the salt is left behind. Reverse osmosis plants usually use less energy than methods that involve heating.
While reverse osmosis is efficient, it needs maintenance. Things like calcium, bacteria, viruses, and tiny particles can clog or damage the membranes. To prevent this, chemicals are added, and the membranes need to be cleaned regularly. This cleaning process can sometimes release contaminated water back into the ocean, which can be risky for marine habitats.
Freeze-Thaw Desalination
This method uses freezing to separate fresh water from salty water. When salty water freezes, the pure water turns to ice, leaving the salt behind.
Solar Evaporation
This method copies nature's water cycle. The sun heats the seawater, causing it to evaporate. The water vapor then cools and condenses onto a surface, becoming fresh water.
Electrodialysis Reversal
This method uses electricity to move the salts through a special membrane, leaving fresh water behind.
Costs of Desalination
The cost of desalination depends on many things: how big the plant is, what type of plant it is, where it's located, the type of water it treats, labor costs, energy prices, and how the salty waste is disposed of.
Desalinating seawater is generally more expensive than getting fresh water from rivers, groundwater, or recycling water. However, these other options are not always available. In 2013, the cost of desalinated water ranged from about US$0.45 to US$1.00 per cubic meter. More than half of this cost comes from energy, and energy prices can change a lot.
In some developing countries, untreated fresh water can cost as much as US$5 per cubic meter.
| Method | Cost (US$/liter) |
|---|---|
| Passive solar (30.42% energy efficient) | 0.034 |
| Passive solar (improved single-slope, India) | 0.024 |
| Passive solar (improved double slope, India) | 0.007 |
| Multi Stage Flash (MSF) | < 0.001 |
| Reverse Osmosis (Concentrated solar power) | 0.0008 |
| Reverse Osmosis (Photovoltaic power) | 0.000825 |
| Area | Water Use Litre/person/day |
Desalinated Water Cost US$/person/day |
|---|---|---|
| US | 378 | 0.38 |
| Europe | 189 | 0.19 |
| Africa | 57 | 0.06 |
| UN recommended minimum | 49 | 0.05 |
Desalination plants carefully control pressure, temperature, and salt levels to work as efficiently as possible. Using nuclear power for desalination might be a cost-effective option for very large plants.
In 2014, desalination plants in Israel were making water for less than US$0.40 per cubic meter. As of 2006, Singapore was desalinating water for US$0.49 per cubic meter.
Images for kids
-
A diagram of a typical desalination plant using reverse osmosis.
See also
 In Spanish: Desalinización para niños
In Spanish: Desalinización para niños