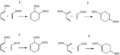Diels–Alder reaction facts for kids
| Diels–Alder reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Named after | Otto Diels Kurt Alder |
||||||||
| Reaction type | Cycloaddition | ||||||||
| Reaction | |||||||||
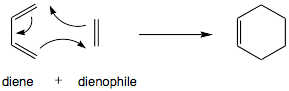
|
|||||||||
| Identifiers | |||||||||
| RSC ontology ID | RXNO:0000006 |
||||||||
The Diels–Alder reaction (DA) is a special chemical reaction. It happens between two different organic compounds. This reaction helps these compounds join together. They form a new compound with a six-sided shape. This new compound is called a cyclohexene.
Imagine one compound has two double bonds. These bonds are separated by one carbon atom. This is called a conjugated diene. It joins with another compound that has at least one double bond. This second compound is an alkene. Together, they create the cyclohexene, which looks like a ring.
Contents
Discovery of the Diels–Alder Reaction
This amazing reaction was found by two scientists. Their names were Otto Diels and Kurt Alder. They discovered it in 1928. Their work was very important.
In 1950, they won the Nobel Prize in Chemistry. This award was for their discovery of the Diels–Alder reaction.
Why the Diels–Alder Reaction is Useful
The DA reaction is very helpful for chemists. It allows them to make cyclohexenes easily. It does not need a lot of energy.
Cyclohexenes are important building blocks. They are used to create complex organic molecules. These molecules are used in many things. For example, one of the first uses was to make insecticides. These are chemicals that kill insects.
How the Reaction Works
The DA reaction creates a new ring-shaped compound. This ring has six sides. It forms when a compound with two double bonds (a diene) meets another compound with at least one double bond (an alkene).
Sometimes, the atoms in the new ring are not all carbon. This can still be a Diels–Alder reaction.
Reversible Reactions
Some Diels–Alder reactions can go both ways. This means they are reversible. The new ring can also break apart. When the ring breaks, it is called a retro-Diels–Alder reaction. Scientists often see these broken compounds when they study them. They use a method called mass spectrometry.
The "Mona Lisa" of Chemistry
Some chemists call the Diels–Alder reaction the 'Mona Lisa' of organic reactions. The Mona Lisa painting has a simple smile. But it might be more complex than it seems. In the same way, this reaction might be more complex than scientists know right now.
Helping the Reaction Happen
Certain chemicals can speed up the Diels–Alder reaction. These chemicals are called catalysts. Examples include Lewis acids. Some common Lewis acids are AlCl3 and ZnCl2. They help the reaction happen faster and more easily.
Images for kids
See also
 In Spanish: Reacción de Diels-Alder para niños
In Spanish: Reacción de Diels-Alder para niños