Silver sulfide facts for kids
Quick facts for kids Silver sulfide |
|
|---|---|
 |
|
 |
|
| IUPAC name | Silver(I) sulfide |
| Other names | Silver sulfide Argentous sulfide |
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES | S(Ag)Ag |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Grayish-blackish crystal |
| Odor | Odorless |
| Density | 7.234 g/cm3 (25 °C) 7.12 g/cm3 (117 °C) |
| Melting point | |
| 6.21·10−15 g/L (25 °C) | |
|
Solubility product (Ksp)
|
6.31·10−50 |
| Solubility | Soluble in aq. HCN, aq. citric acid with KNO3 Insoluble in acids, alkalies, aqueous ammoniums |
| Structure | |
| Crystal structure | Monoclinic, mP12 (α-form) Cubic, cI8 (β-form) Cubic, cF12 (γ-form) |
| Space group | Im3m, No. 229 (α-form) P21/n, No. 14 (β-form) Fm3m, No. 225 (γ-form) |
| 2/m (α-form) 4/m 3 2/m (β-form, γ-form) |
|
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−32.59 kJ/mol |
| Standard molar entropy S |
143.93 J/mol·K |
| Specific heat capacity, C | 76.57 J/mol·K |
| Hazards | |
| Main hazards | May cause irritation |
| NFPA 704 |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Silver sulfide is a black, solid chemical compound. Its chemical formula is Ag2S. It is made of two elements: silver and sulfur. You might have seen it before as the dark coating, called tarnish, that forms on old silverware or silver jewelry. It is the only known chemical compound made of just silver and sulfur.
It is useful in photography because it reacts to light. It is also a very strong material that does not dissolve in water or most other liquids, although strong acids can break it down.
Contents
How Silver Sulfide Forms
Silver sulfide forms naturally on silver objects. This happens when silver reacts with hydrogen sulfide gas in the air. This gas often smells like rotten eggs. The reaction creates a thin black layer on the surface of the silver. This layer is called a patina. Interestingly, this black layer actually protects the silver underneath from getting damaged further.
Sometimes, tiny "whiskers" of silver sulfide can grow on silver parts used in electronics. This usually happens in places where the air is damp and has a lot of sulfur, like in sewage treatment plants or paper mills.
Shapes and Minerals
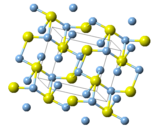
Silver sulfide can change its internal crystal shape depending on the temperature. This is called polymorphism.
Types of Crystals
- Acanthite (α-form): At room temperature (below 179 °C), it forms a crystal shape called monoclinic. This form is found in nature as a mineral called acanthite. It is an important ore, which means people mine it to get silver.
- Argentite (β-form): When it gets hotter (above 180 °C), it changes to a cubic shape. This form is called argentite.
- Gamma form (γ-form): At very high temperatures (above 586 °C), it changes shape again.
The forms that exist at high temperatures can conduct electricity.
Bending Without Breaking
Most stone-like materials or crystals will shatter if you hit them or try to bend them. However, silver sulfide is different. It has a property called ductility. This means it can stretch and bend without breaking, much like a metal wire.
Why is it flexible?
Scientists have found that silver sulfide can be squashed or pulled quite a lot. This happens because of how the atoms are arranged inside. The layers of atoms can slide past each other easily. The bonds holding the atoms together are strong enough to keep it in one piece, but flexible enough to let it change shape. This makes it very resistant to cracking.
History of Discovery
In 1833, the famous scientist Michael Faraday made an interesting discovery about silver sulfide. He noticed that it conducted electricity better as it got hotter. This is the opposite of how normal metals work (metals usually conduct worse when hot). This was the very first time someone discovered a semiconductor. Semiconductors are materials used today in all computers and phones.
Is silver sulfide dangerous to humans?
Silver sulfide poses minimal danger to humans under normal conditions. You can handle tarnished silver safely. The main annoyance is black stains on your fingers.
The only plausible way to experience adverse health effects (like argyria) is through long-term, high-level inhalation of silver dusts, which is confined to specific industrial workplaces without proper safety controls (ventilation, respirators). While disfiguring and permanent, argyria is not associated with organ toxicity, cancer, or increased mortality.
When using silver polish (which contains chemicals to remove the sulfide), wear gloves to protect your skin from the polish's harsh ingredients, not from the tarnish itself.
Related pages
See also
 In Spanish: Sulfuro de plata para niños
In Spanish: Sulfuro de plata para niños
 | Jewel Prestage |
 | Ella Baker |
 | Fannie Lou Hamer |


