Nonmetallic material facts for kids
A nonmetallic material, or simply a nonmetal, is any material that is not a metal. Think of things like wood, plastic, or ceramic – these are common nonmetals you see every day. Scientists and engineers use the term "nonmetal" in different ways, depending on what they are studying.
In chemistry, nonmetals are specific chemical elements on the periodic table that don't act like metals under normal conditions. In physics, nonmetals are materials where electrons cannot move around easily. This means they don't conduct electricity well. Interestingly, in astronomy, the definition is very different: only hydrogen and helium are considered nonmetals!
Sometimes, a nonmetal can change into a metal, or a metal into a nonmetal. This usually happens because of big changes in temperature or pressure. For example, a strong electric field can make a small part of a semiconductor device act like a nonmetal. Nonmetals also have unique properties, like piezoelectricity, which means they can create electricity when squeezed.
Contents
How Scientists Define Nonmetals

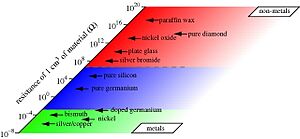
Scientists often define nonmetals by how their electrons behave. Imagine electrons in a material having different energy levels, like steps on a ladder. In a nonmetal, there's a big "gap" between the energy levels that electrons usually occupy and the levels where they could move freely. This "gap" is called a band gap.
Because of this gap, electrons in nonmetals can't easily jump to higher energy levels and move around. This is why nonmetals usually don't conduct electricity well. In contrast, metals have electrons that can move freely, making them good conductors.
This idea helps us understand why materials like boron are nonmetals, while compounds like titanium nitride are metals. Scientists use special tests and computer calculations to measure this "band gap" and figure out if a material is a nonmetal.
Nonmetals in Everyday Life and Industry
In everyday life and industries like metallurgy (the study of metals), people often think of metals as materials that can be easily shaped, like steel or aluminium alloys. Everything else, like plastics or wood, is often called a nonmetal.
While this is a common way to think, it's not always perfectly accurate. For example, some special plastics, called conducting polymers, can actually conduct electricity like metals! Also, some semiconductor materials, which are usually nonmetals, can start acting like metals if you add certain impurities, called dopants, to them.
Nonmetals on the Periodic Table
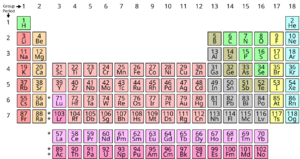
In chemistry, nonmetals are specific chemical elements found on the periodic table. These are elements that don't show typical metallic properties under normal conditions. You can usually find them on the top right side of the periodic table.
Elements on the left and in the middle of the periodic table are generally metals, like iron or gold. Some elements in between are called metalloids because they have properties of both metals and nonmetals.
Scientists also use the idea of nonmetals when they add small amounts of one element (called a dopant) to another material. For example, adding certain nonmetals can make steel stronger.
Nonmetals in Space (Astronomy)
In astronomy, the word "metal" has a very special meaning. Astronomers call all elements heavier than hydrogen and helium "metals." This means that in space, only hydrogen and helium are considered nonmetals!
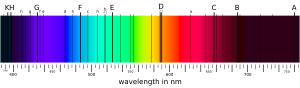
This unique definition comes from history. In the early 1800s, scientists like Joseph von Fraunhofer studied the light from the Sun. They saw dark lines in the Sun's spectrum, called Fraunhofer lines. Later, scientists realized these lines were caused by elements in the Sun's atmosphere absorbing light.
The strongest lines they saw came from elements like sodium and iron, which are metals on Earth. So, early astronomers started calling all elements they could detect, besides hydrogen and helium, "metals." Even today, astronomers use this term, so when they talk about a star's "metallicity," they mean how much of everything besides hydrogen and helium it contains.
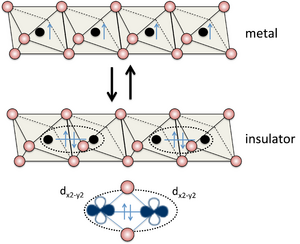
When Nonmetals Can Act Like Metals
Sometimes, a material can switch between being a metal and a nonmetal. This is called a metal–insulator transition. For example, under extreme pressure, hydrogen, which is normally a nonmetal gas, can turn into metallic hydrogen, which conducts electricity.
These changes can also happen in tiny parts of semiconductor devices. For instance, in a field-effect transistor, an electric field can create a small area where electrons can't move, making that part act like a nonmetal.
Special Qualities of Nonmetals
Nonmetals have many interesting and useful properties. For example, diamond, a nonmetal, is the hardest known natural material. Another nonmetal, molybdenum disulfide, is used as a lubricant in space because it's very slippery.
Because electrons in nonmetals don't move freely, they have some special characteristics:
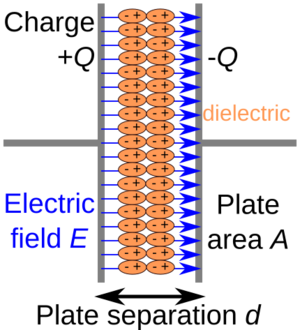
- Dielectric polarization: This means they can store electrical energy, like in a capacitor. The material's tiny electrical parts (dipoles) line up when an electric field is applied.
- Electrostriction: Some nonmetals can change their size slightly when an electric field is applied to them.
- Flexoelectricity: This is when bending or stretching a nonmetal can create an electric charge. It helps explain how static electricity is made when you rub things together.
- Piezoelectricity: Certain nonmetals can produce electricity when you squeeze or press them. They can also change shape when electricity is applied.
- Decreased resistance with temperature: Unlike metals, many nonmetals become better at conducting electricity as they get warmer. This is because more electrons gain enough energy to move.
- Photoconductivity: Some nonmetals conduct electricity better when light shines on them. The light gives electrons enough energy to start moving.
- Transmitting electric fields: Nonmetals can allow electric fields to pass through them, which is important for devices like capacitors. Metals, however, block electric fields.
See Also
- Abundance of the chemical elements
- Electrical conduction
- List of data references for chemical elements
- List of materials properties
- List of states of matter
- Metallicity distribution function
- Metal–insulator transition
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |