Solution facts for kids

In chemistry, a solution is a special kind of mixture. It's where two or more substances are perfectly mixed together. You can't easily tell them apart.
The substances that get dissolved are called solutes. The substance that does the dissolving is called the solvent.
Think of it like making a drink. If you stir table salt or sugar into water, the salt or sugar is the solute. The water is the solvent. The salty or sugary water you get is the solution!
Solutions aren't just liquids. Gases can dissolve in liquids, like oxygen in water for fish. Liquids can dissolve in other liquids, like ethanol in water. Even gases can dissolve in other gases, like the air we breathe.
The amount of solute in a solvent changes how strong the solution is. This is called its concentration. A solution with a lot of solute is called a concentrated solution. One with less solute is a dilute solution.
Some solid solutions exist too. Alloys are a good example. Brass, for instance, is a solid solution of copper and zinc metals. When water is the solvent, we call it an "aqueous solution".
Contents
What Are the Different Kinds of Solutions?
Solutions are always homogeneous. This means everything is mixed evenly. You can't see separate parts.
Gas Solutions
When the solvent is a gas, other gases or vapors can dissolve in it. The most common example is air. Air is a solution of oxygen and other gases dissolved in nitrogen.
Liquid Solutions
Liquids are great at dissolving things. They can dissolve gases, other liquids, and solids.
- Gas in liquid: Fish breathe oxygen that's dissolved in water.
- Liquid in liquid: Alcoholic beverages are solutions of ethanol dissolved in water.
- Solid in liquid: When you stir sucrose (sugar) into water, you make sugar water.
Solid Solutions
Sometimes, a solid can be the solvent. Gases, liquids, or other solids can dissolve in it.
- Gas in solid: Hydrogen gas can dissolve in some metals, like palladium.
- Liquid in solid: Mercury can dissolve in gold to form an amalgam.
- Solid in solid: Steel is a solution of carbon atoms mixed into iron. Alloys like bronze are also solid solutions.
How Much Can Dissolve? (Solubility)
The ability of one substance to dissolve in another is called solubility.
- When two liquids can completely mix, they are miscible. Think of water and vinegar.
- If two substances can never mix to form a solution, they are immiscible. Oil and water are a good example.
Usually, if you make a liquid solvent warmer, it can dissolve more of a solid solute. For example, sugar dissolves faster in hot tea than in cold tea. But for most gases, it's the opposite. Gases dissolve better in colder liquids. That's why cold soda keeps its fizz better than warm soda.
How Solutions Change Things
When you make a solution, the physical properties of the substances can change. Things like the melting point and boiling point might be different.
The amount of solute in a solution is called its concentration. There are different ways to measure this. For example, a 50% ethanol solution means half of it is ethanol and half is water.
What Makes Liquid Solvents Special?
Most solvents we use in chemistry are molecular liquids. They can be divided into two main types:
- Polar solvents: These have molecules with a slight positive and a slight negative end. Water is a great example.
- Non-polar solvents: These have molecules that are more balanced.
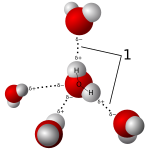
Water is a very common solvent because it's polar. It can also form special bonds called hydrogen bonds.
Salts dissolve well in polar solvents like water. When salt dissolves, it breaks into tiny charged pieces called ions. These ions are attracted to the charged ends of the water molecules. This process is called hydration when water is the solvent. Solutions of dissolved salts are called electrolytes.
Polar solutes dissolve best in polar solvents. This is why ethanol (alcohol) mixes perfectly with water. Non-polar solutes dissolve best in non-polar solvents. This is why oil and grease mix easily with each other, but not with water.
You can see this with an oil spill in the ocean. The oil doesn't dissolve in the ocean water. Instead, it floats on top because oil and water are immiscible.
Related Topics
See also
 In Spanish: Solución para niños
In Spanish: Solución para niños
- Total dissolved solids

