Diatomic molecule facts for kids
Diatomic molecules are tiny particles (called molecules) made of only two atoms. These two atoms can be the same kind of chemical element or different kinds.
If a diatomic molecule has two atoms of the same element, like hydrogen (H
2) or oxygen (O
2), it's called a homonuclear molecule. The atoms in these molecules share their electrons equally, making the bond between them non-polar.
If a diatomic molecule has two different atoms, like carbon monoxide (CO) or nitric oxide (NO), it's called a heteronuclear molecule.
Contents
Common Diatomic Elements
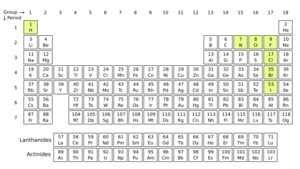
Only a few elements naturally form stable homonuclear diatomic molecules as gases at normal temperatures and pressures (like in a typical lab). These are:
- Hydrogen (H
2) - Nitrogen (N
2) - Oxygen (O
2) - Fluorine (F
2) - Chlorine (Cl
2)
The noble gases (like helium and neon) are also gases at normal conditions, but they exist as single atoms, not pairs.
At slightly warmer temperatures, bromine (Br
2) and iodine (I
2) also become diatomic gases. All halogens (a group of elements) can form diatomic molecules, except for astatine and tennessine, which are less certain.
Other elements can form diatomic molecules when they are heated up and turn into a gas. For example, heating phosphorus creates diphosphorus (P
2), and sulfur vapor is mostly disulfur (S
2). Even some metals like dilithium (Li
2) and disodium (Na
2) can exist as diatomic molecules in the gas phase.
Heteronuclear Molecules
Many different elements can combine to form heteronuclear diatomic molecules. These are always chemical compounds made of two different elements.
Some common examples of heteronuclear diatomic gases are:
- Carbon monoxide (CO)
- Nitric oxide (NO)
- Hydrogen chloride (HCl)
Many compounds that are usually solid or liquid at room temperature can also form diatomic molecules when they are heated and turn into a gas. For example, gaseous MgO and SiO.
Where Are Diatomic Molecules Found?
Scientists have found hundreds of different diatomic molecules in nature, in labs, and even in outer space!
About 99% of the Earth's atmosphere is made up of two types of diatomic molecules:
- Nitrogen (N2) makes up about 78% of the air.
- Oxygen (O2) makes up about 21% of the air.
While hydrogen (H2) is rare in Earth's atmosphere, it is the most common diatomic molecule in the entire universe!
Molecular Shape
All diatomic molecules have a simple, straight-line shape. They are described by just one measurement: the distance between the two atoms, called the bond length.
The atoms in diatomic molecules can be connected by different types of bonds:
- Diatomic nitrogen (N2) has a triple bond.
- Diatomic oxygen (O2) has a double bond.
- Diatomic hydrogen (H2), fluorine (F2), chlorine (Cl2), iodine (I2), and bromine (Br2) all have single bonds.
Historical Importance
Diatomic elements were very important in the 1800s for helping scientists understand what elements, atoms, and molecules really were. Back then, some scientists like John Dalton thought all elements were made of single atoms. This led to confusion about how much atoms weighed and what the formulas for compounds were. For example, Dalton thought water was HO, not H2O.
In 1805, scientists Gay-Lussac and von Humboldt showed that water was made of two parts hydrogen and one part oxygen. Then, in 1811, Amedeo Avogadro came up with the correct idea: that elements like hydrogen and oxygen existed as diatomic molecules (H2 and O2). This helped explain how they combined to form water (H2O).
However, many scientists didn't accept Avogadro's ideas right away. It wasn't until 1860, at a big meeting of scientists called the Karlsruhe Congress, that Cannizzaro brought Avogadro's ideas back into focus. This helped create a correct table of atomic weights, which was a huge step forward and led to the discovery of the periodic law by Dmitri Mendeleev.
Excited States and Energy
Diatomic molecules usually exist in their lowest energy state. But if they get hit by energetic particles (like electrons) or absorb light, they can jump to higher energy states. This happens naturally in the aurora (the Northern and Southern Lights), where nitrogen and oxygen molecules get excited and then release light as they return to their normal state. This light emission is called fluorescence.
Molecules can also have different types of energy from their movements:
- Translational energy is the energy of the whole molecule moving from one place to another.
- Rotational energy is the energy of the molecule spinning around.
- Vibrational energy is the energy of the atoms in the molecule wiggling back and forth, like they are connected by a spring.
The energy needed to make a molecule vibrate is much greater (about 100 times more) than the energy needed to make it spin faster.
Easy Ways to Remember Diatomic Elements
There are some fun ways to remember the common diatomic elements (H2, O2, F2, Br2, I2, N2, Cl2):
- BrINClHOF (pronounced "Brinklehof")
- HONClBrIF (pronounced "Honkelbrif")
- Never Have Fear of Ice Cold Beer (Nitrogen, Hydrogen, Fluorine, Oxygen, Iodine, Chlorine, Bromine)
See also
 In Spanish: Molécula diatómica para niños
In Spanish: Molécula diatómica para niños
- Symmetry of diatomic molecules
- AXE method
- Octatomic element
- Covalent bond
- Industrial gas
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |