Mixtures and solutions facts for kids
Mixtures and solutions are special types of combinations that form when you put different things together. Imagine mixing ingredients for a recipe – sometimes they blend perfectly, and sometimes you can still see each part!
Contents
What's the Difference Between Mixtures and Solutions?
When you combine two or more substances, you create either a mixture or a solution. The main difference is how well they blend.
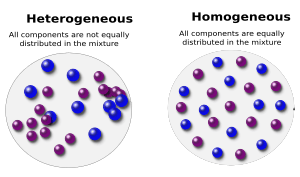
A mixture is a combination where the different parts don't chemically join. You can often see the different parts, or they might settle over time. For example, if you mix sand and water, you can clearly see the sand at the bottom and the water above it. They keep their own properties.
A solution is a special kind of mixture where one substance completely dissolves into another. This means the parts are spread out evenly, and you can't easily tell them apart just by looking. Think about stirring sugar into water. The sugar seems to disappear, creating a clear sugar-water solution. Solutions are also called homogeneous because they look the same throughout. If you take a tiny bit from the top or the bottom, it will have the same amount of sugar and water.
Exploring Mixtures
In chemistry, a mixture is made when two or more simple substances come together. These substances can be elements (like gold) or compounds (like water). Mixtures can be made of liquids, solids, or gases.
It's important to remember that a mixture is different from a compound. In a compound, atoms are chemically linked together, like how hydrogen and oxygen atoms join to make water. In a mixture, the substances are just mixed, not chemically bonded. For example, a mixture of hydrogen gas and nitrogen gas is just that – hydrogen and nitrogen. It's not ammonia, which is a compound made from those same elements.

Mixtures can be divided into two main types:
- Heterogeneous mixtures: These are mixtures where you can easily see the different parts. The substances are not spread out evenly. Think of a salad – you can see the lettuce, tomatoes, and dressing separately.
- Homogeneous mixtures: These mixtures look the same all the way through. The substances are evenly mixed, and you can't easily tell them apart. An example is clean air, which is a mix of different gases like nitrogen and oxygen, but it looks uniform.
A special type of mixture is called a colloid. In a colloid, one substance is spread out very finely within another. The particles are bigger than in a solution but too small to settle out quickly. Milk is a good example of a colloid.
If one substance in a mixture completely dissolves into another, it forms a solution. If it doesn't dissolve, it's called a suspension. For instance, if you put sugar in water, it forms a mixture, then dissolves to make a solution. If you put sand in water, it's a suspension because the sand doesn't dissolve.
Even solids can be mixtures! Alloys are mixtures of different metals, like brass (a mix of copper and zinc). Many kinds of soil and rock are also mixtures of different minerals.
Examples of Mixtures
Heterogeneous Mixture Examples
These mixtures have parts you can clearly see or separate.
- Salt and pepper mixed together.
- A basket full of different kinds of apples.
- Ice cubes floating in a soda.
- Water and sand stirred together.
- Water and oil, which separate into layers.
Homogeneous Mixture Examples
These mixtures look uniform throughout.
- Coffee (without grounds).
- Brass (a metal alloy).
- Liquefied petroleum gas (LPG).
- Wine.
- Air (a mixture of gases).
Understanding Solutions
In chemistry, a solution is a special kind of homogeneous mixture. This means its parts are perfectly blended and look the same everywhere.
In a solution, we have two main parts:
- The solute is the substance that gets dissolved.
- The solvent is the substance that does the dissolving.
A common example is making salt water by dissolving table salt (NaCl) in water. Here, salt is the solute, and water is the solvent.
Solutions aren't just liquids! Gases can dissolve in liquids, like carbon dioxide in soda or oxygen in water (which fish need to breathe). Liquids can dissolve in other liquids, and gases can even dissolve in other gases.
The amount of solute in a solvent tells us the solution's concentration. If there's a lot of solute, it's a concentrated solution. If there's less solute, it's a dilute solution.
Solid solutions also exist. Alloys are great examples, like brass, which is a solid solution of copper and zinc.
Related Pages
Images for kids
-
Red wine in a glass, a homogeneous mixture.
 | Tommie Smith |
 | Simone Manuel |
 | Shani Davis |
 | Simone Biles |
 | Alice Coachman |