Osteogenesis imperfecta facts for kids
Quick facts for kids Osteogenesis imperfecta(OI) |
|
|---|---|
| Classification and external resources | |
| Pronunciation | /ˌɒstioʊˈdʒɛnəsɪs ˌɪmpɜːrˈ[unsupported input][unsupported input]/ oss-TEE-oh-JEN-ə-SISS-_ |
| Synonyms | Brittle bone disease, Lobstein syndrome, fragilitas ossium, Vrolik disease, osteopsathyrosis idiopathica |
| Specialty | Pediatrics, medical genetics, orthopedics |
| Patient UK | Osteogenesis imperfecta (OI) |
Osteogenesis imperfecta (IPA: /ˌɒstioʊˈdʒɛnəsɪs ˌɪmpɜːrˈ[unsupported input][unsupported input]/), often called brittle bone disease, is a group of genetic disorders. These conditions make bones break very easily. The problems can be mild or severe, affecting bones and other body parts.
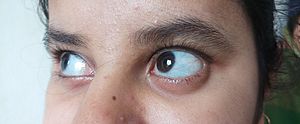
Common signs of OI include the whites of the eye looking blue, being shorter than average, having loose joints, and hearing loss. Some people might also have breathing issues or problems with their teeth. In severe cases, there can be serious complications like tears in major arteries (like the aorta) or problems with the ribcage affecting breathing.
OI is usually caused by a problem with connective tissue, specifically with type I collagen. This happens because of changes (mutations) in the COL1A1 or COL1A2 genes. These changes can be passed down from parents or happen randomly. There are four main types: type I (mildest), type IV (moderate), type III (severe and deforming), and type II (most severe, often fatal at birth). As of 2021, 21 types of OI are known, caused by 19 different genes. Doctors often diagnose OI based on symptoms, and it can be confirmed with DNA testing.
There is no cure for OI, but most people with it can live a long life. Death in childhood from OI is rare. Many adults with OI can live independently despite their challenges. Living a healthy lifestyle with exercising, eating well (with enough vitamin D and calcium), and not smoking can help prevent fractures. Genetic counseling can help families understand the risks of passing OI to their children.
Treatment for OI includes caring for broken bones, managing pain, physical therapy, and using mobility aids like leg braces or wheelchairs. Sometimes, metal rods are put into long bones (like the femur) to make them stronger. Medicines called bisphosphonates can also help make bones denser, especially in children.
OI is a rare genetic disease, affecting about 1 in 15,000 to 20,000 people. Type I is the most common. The outcome depends on the specific type. With surgery, some people with more severe OI can learn to walk. The condition has been known since ancient times. The name osteogenesis imperfecta means "imperfect bone formation" in Latin.
Contents
- What are the Signs of Osteogenesis Imperfecta?
- How is Osteogenesis Imperfecta Classified?
- What Causes Osteogenesis Imperfecta?
- How is Osteogenesis Imperfecta Diagnosed?
- How is Osteogenesis Imperfecta Treated?
- Can Osteogenesis Imperfecta be Prevented?
- What is the Outlook for People with Osteogenesis Imperfecta?
- How Common is Osteogenesis Imperfecta?
- History of Osteogenesis Imperfecta
- OI in Animals
- See also
What are the Signs of Osteogenesis Imperfecta?
Bone and Joint Issues
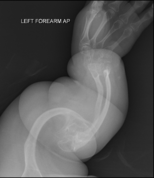
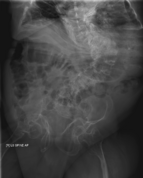
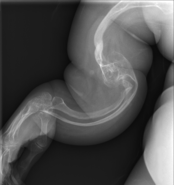
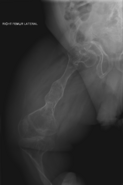
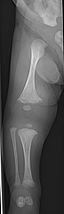
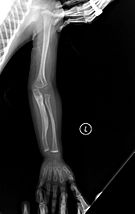
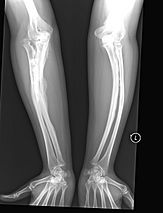
The main sign of osteogenesis imperfecta (OI) is bones that are weak and have low mineral density. All types of OI affect the bones. In moderate and severe OI, long bones might be bowed (curved).
Weak bones break easily. One study found that children with OI had about 5.8 fractures per year if not treated. Fractures usually happen less often after puberty. But they can increase again in women after menopause and in men between 60 and 80 years old.
Joint hypermobility (very flexible joints) is also common in OI. This is because the genes involved are similar to those that cause some types of Ehlers–Danlos syndrome.
Hearing Problems
About half of adults with OI experience significant hearing loss by age 50. This is much earlier than in the general population. Hearing loss can start in the 20s, 30s, or 40s. It can be conductive (sound not reaching the inner ear), sensorineural (nerve damage), or a mix of both.
Hearing loss is most common in type I OI. It is less common in types III and IV. OI can also affect balance, but this is rare.
Other Body Systems
While OI mainly affects bones, it can also impact almost all other organs in the body. This is especially true for types caused by type I collagen gene mutations.
Type I collagen is found in the circulatory and respiratory systems. This includes the heart valves, vasculature (blood vessels), and lungs. So, heart problems like aortic insufficiency or aortic aneurysm can sometimes occur with OI.
Breathing problems are a major cause of death in OI. This is often due to issues with the thoracic wall (ribcage). Respiratory tract infections like pneumonia are also more dangerous for people with OI.
OI, especially severe type III, can also affect the digestive system. People with OI may have abdominal pain and chronic constipation. Constipation is very common and can lead to feeling full and not wanting to eat, which might cause malnutrition.
How is Osteogenesis Imperfecta Classified?
There are two main ways to classify OI today. The first, created by David Sillence in 1979, groups patients into four types based on their symptoms. The second system adds new numbered types as new genetic causes are found. So, a person with OI might have both a "clinical" type and a "genetic" type.
Type I is the most common. About 90% of cases are due to mutations in COL1A1 or COL1A2 genes. Symptoms can vary greatly, even within the same family.
As of 2021, 21 types of OI have been identified:
| Sillence's four types | ||||||
|---|---|---|---|---|---|---|
| Type | Description | Gene | OMIM | Mode of inheritance | Incidence | Defined |
| I | mild | Null COL1A1 allele | 166200 | autosomal dominant, 34% de novo | 1 in 30,000 | 1979 |
| II | lethal in the perinatal period | COL1A1, COL1A2 | 166210 | autosomal dominant, ≈100% de novo | 1 in 40,000 to 1 in 100,000 | |
| III | severe, progressive and deforming | COL1A1, COL1A2 | 259420 | autosomal dominant, 85% de novo | 1 in 60,000 | |
| IV | variable and deforming, but usually with normal sclerae | COL1A1, COL1A2 | 166220 | autosomal dominant, 50% de novo | 1 in 30,000 | |
| Genetically defined types | ||||
|---|---|---|---|---|
| Type | Gene | OMIM | Mode of inheritance | Defined |
| V | IFITM5 | 610967 | autosomal dominant | 2000 |
| VI | SERPINF1 | 613982 | autosomal recessive | 2002 |
| VII | CRTAP | 610682 | autosomal recessive | 2006 |
| VIII | LEPRE1 | 610915 | autosomal recessive | 2007 |
| IX | PPIB | 259440 | autosomal recessive | 2009 |
| X | SERPINH1 | 613848 | autosomal recessive | 2010 |
| XI | FKBP10 | 610968 | autosomal recessive | 2010 |
| XII | SP7 | 613849 | autosomal recessive | 2010 |
| XIII | BMP1 | 614856 | autosomal recessive | 2012 |
| XIV | TMEM38B | 615066 | autosomal recessive | 2012 |
| XV | WNT1 | 615220 | autosomal recessive | 2013 |
| XVI | CREB3L1 | 616229 | autosomal recessive | 2013 |
| XVII | SPARC | 616507 | autosomal recessive | 2015 |
| XVIII | TENT5A | 617952 | autosomal recessive | 2018 |
| XIX | MBTPS2 | 301014 | X-linked recessive | 2016 |
| XX | MESD | 618644 | autosomal recessive | 2019 |
| XXI | KDELR2 | 619131 | autosomal recessive | 2020 |
Sillence's Four Types of OI
Sillence's four types describe the common symptoms. They can also refer to genetic types caused by mutations in the COL1A1 or COL1A2 genes.
Type I OI: Mildest Form
In type I OI, the body makes enough collagen, but its quality is normal. Bones break more easily than usual, but not as often as in more severe types. People might have mild scoliosis (curved spine) or loose joints. Blue sclerae (whites of the eyes) are common, and hearing loss can occur. People with type I are usually only slightly shorter than average. Sometimes, type I OI isn't noticed until adulthood because the symptoms are so mild.
Some doctors divide type I into I-A (no opalescent teeth) and I-B (with opalescent teeth). People with type I usually have a normal lifespan.
Type II OI: Most Severe Form
Type II OI is caused by a very serious defect in collagen. Most babies with type II OI die shortly after birth, often due to respiratory failure or skull fractures. Many are born with multiple broken bones. Babies with type II also have severe breathing problems and very deformed bones. Survival past the first year is very rare and usually requires a breathing machine.
Type II is known as the "lethal perinatal" form. This means it's not possible to live into adulthood with this type. Sometimes, severe type III cases are first thought to be type II. But if the baby survives for a longer time, the diagnosis changes to type III.
Type III OI: Severe and Deforming
In type III OI, the body makes enough collagen, but it's not good quality. It can be hard to tell type III from type IV. However, type III gets worse over time and often causes a "triangular" face shape. Babies with type III often have blue sclerae that turn white as they get older. Blue sclerae are less common in type IV.
Type III OI causes very fragile bones that break easily, sometimes even before birth. People with type III can have hundreds of fractures in their lifetime. They often develop early scoliosis that worsens until puberty. Dwarfism is common, with adult height often less than 4 feet or 120 centimetres. Loose joints and breathing problems (due to small rib cage volume) are also common.
Because of severe bone issues, people with type III are more likely to have neurological problems or seizure disorders. Pressure on the brainstem (called basilar invagination) can be life-threatening.
Type IV OI: Variable Severity
Type IV OI also involves enough collagen, but it's not good quality. This type has a wide range of severity and doesn't fit into type I or III. While it was once thought that people with type IV had normal sclerae, modern classification allows for blue sclerae in some cases.
In type IV, bone deformities can be mild to severe. Bones break easily, especially before puberty. Dwarfism is common, and vertebral collapse and scoliosis can occur. Hearing loss is possible but not as common as in type I. Type IV is often called "variable" OI because its severity can differ even within the same family.
Before puberty, people with type IV OI tend to have about one fracture per year. This is less than the three fractures per year seen in severe type III OI.
Like type I, type IV can be split into IV-A (no opalescent teeth) and IV-B (with opalescent teeth).
Genetically Defined Types (Types V–XXI)
As of 2020, there are fifteen genetically defined types of OI:
- Type V has similar symptoms to type IV. It can be identified by a "mesh-like" look in a bone biopsy under a microscope. Type V also has a "V triad" of signs: an opaque band near growth plates (seen on X-ray), very large calluses (bony repair tissue) at fracture sites, and hardening of the forearm membrane, which can make it hard to turn the wrist. This type is caused by mutations in the IFITM5 gene.
- Type VI has symptoms like type III. It's caused by a mutation in the SERPINF1 gene.
- Type VII is caused by a mutation in the CRTAP gene. Its symptoms are similar to types II and III.
- Type VIII is caused by a mutation in the LEPRE1 gene. Its symptoms are similar to types II and III.
- Type IX is caused by mutations in the PPIB gene.
- Type X is caused by a mutation in the SERPINH1 gene.
- Type XI is caused by mutations in FKBP10 gene. These mutations reduce the amount of procollagen.
- Type XII is caused by a mutation in SP7 gene. This leads to bone deformities, fractures, and delayed tooth eruption.
- Type XIII is caused by a mutation in the bone morphogenetic protein 1 (BMP1) gene. This causes repeated fractures, high bone mass, and hypermobile joints.
- Type XIV is caused by mutations in the TMEM38B gene. This causes repeated fractures and osteopenia (low bone density).
- Type XV is caused by mutations in the WNT1 gene.
- Type XVI is caused by mutations in the CREB3L1 gene. It causes fractures of ribs and long bones before birth, weak bones, and blue sclerae. It looks like type II or III.
- Type XVII is caused by a mutation in the SPARC gene. This leads to severe disease with flat vertebrae, wheelchair use, and repeated fractures.
- Type XVIII is caused by a mutation in the FAM46A gene. It causes bowed long bones at birth, extra bones in the skull, blue sclerae, and many fractures early in life.
- Type XIX is caused by a mutation in the MBTPS2 gene. This is the only type of OI that is X-linked recessive, meaning it's more common in males.
- Type XX is caused by a mutation in the MESD gene. Early studies suggest it might cause global developmental delay, which is new for OI types.
- Type XXI is caused by a mutation in the KDELR2 gene. It causes symptoms similar to types II and III.
New types are still being discovered as research continues.
What Causes Osteogenesis Imperfecta?
Osteogenesis imperfecta is a group of genetic disorders that all lead to fragile bones. Many different mutations can cause similar symptoms.
The main cause is mutations in the COL1A1 and/or COL1A2 genes. These genes are responsible for making collagen type I. About 90% of people with OI have mutations in one of these two genes. These mutations can lead to problems like stress inside cells, abnormal bone hardening, and issues with how cells interact with each other and with the matrix around them.
Earlier, it was thought that OI was mostly an autosomal dominant disorder (meaning one copy of a changed gene causes the condition). But with cheaper DNA sequencing, doctors have found autosomal recessive forms (meaning two copies of a changed gene are needed). Recessive forms often involve defects in "chaperone proteins." These proteins help collagen form correctly. When they don't work, collagen doesn't fold properly, leading to OI.
Defects in other proteins, like PEDF and BRIL, cause types V and VI OI. These defects lead to bones that don't harden properly, causing them to be brittle. For example, a small change in the IFITM5 gene, which makes BRIL, is directly linked to OI type V.
In the rare type XIX, OI is inherited in an X-linked way. This means the gene is on the X chromosome. This type affects how bone forms. Genetic research is ongoing, and more causes of OI might be found in the future.
Sometimes, OI can appear in siblings even if the parents don't show symptoms. This is due to genetic mosaicism. This means some of a parent's cells (especially germ cells) have the OI gene mutation, but not enough of their other cells do to cause symptoms in the parent.
How is Osteogenesis Imperfecta Diagnosed?
Doctors usually diagnose OI based on medical imaging, like X-rays, and symptoms. In severe OI, X-rays can show bone problems in all limbs and the spine. For mild type I OI, where bone density loss is small, DEXA scans might be needed.
An OI diagnosis can be confirmed with DNA testing or by looking at collagen proteins. However, often, many bone fractures from minor injuries and other signs like blue sclerae are enough for a diagnosis. A skin biopsy can show the structure and amount of type I collagen. While DNA testing can confirm OI, it can't rule it out completely. This is because not all OI-causing mutations are known or tested for yet. Type II OI is often found during pregnancy using ultrasound, as multiple fractures might already be visible. OI can also be detected before birth using amniocentresis.
Genetic Testing for OI
To check for OI, doctors might test the most common genes involved: COL1A1, COL1A2, and IFITM5. If no mutation is found but OI is still suspected, other known OI genes might be tested. Parents can also get tested for gene changes that cause OI.
What Else Could it Be?
It's important to tell OI apart from child abuse. Both can cause many fractures at different healing stages. This can be difficult, especially if there are no other clear signs of OI. This has even led to legal issues in court cases.
Other conditions that can look like OI include rickets and osteomalacia (both caused by poor nutrition). Rare bone syndromes like Bruck syndrome or Ehlers–Danlos syndrome are also considered. Different types of osteoporosis (bone thinning) should also be ruled out.
How is Osteogenesis Imperfecta Treated?
There is no cure for osteogenesis imperfecta. But living a healthy lifestyle, exercising, and avoiding smoking can help prevent fractures. Treatment focuses on caring for broken bones, managing pain, physical therapy, using mobility aids, and sometimes surgery.
It can be hard to tell if a treatment is working because fracture rates can change naturally. Doctors should consider the whole patient, not just how many bones break.
Caring for Broken Bones
Broken bones in people with OI are treated much like anyone else's. OI bones heal at the same rate. However, lightweight materials are used for casts. Heavy casts can cause new fractures in weak bones. After a few weeks, the cast is replaced with a removable brace. All fractures, even small ones, should be treated to prevent problems with healing.
Bone infections are treated with antibiotics and antiseptics, just like in the general population.
Medications for OI
Bisphosphonates
In 1998, a study showed that intravenous pamidronate, a bisphosphonate, helped people with severe OI. It reduced bone pain, prevented new spine fractures, and reduced the number of long-bone fractures.
While oral bisphosphonates are easier to take and cheaper, intravenous ones are generally more effective. Some studies have found them to be similar. In a 2013 study of children with mild OI, oral risedronate increased bone density and reduced non-spine fractures. However, it didn't reduce new spine fractures. A 2016 review found that bisphosphonates seem to improve bone density. But it's not clear if they reduce fractures or improve quality of life.
Bisphosphonates are not as effective at increasing bone density in adults.
Nutritional Supplements
OI is a genetic disorder, not caused by a lack of vitamins or minerals. Supplements cannot cure it. However, people with OI often have very low vitamin D levels. The reason for this is not fully understood. Vitamin D supplements might be recommended until levels return to normal. Low vitamin D can also make bisphosphonates less effective.
Surgery for OI
Any surgery carries more risks for people with OI, especially moderate to severe types. Bone deformities can make it harder to access the airway for breathing tubes. Using and removing mechanical ventilation is also more challenging. During surgery or healing, defective OI collagen can lead to bleeding.
Anesthesia is also a bigger concern. Complications are more likely in type III OI. A unique risk is fractures during surgery from patient movement or airway procedures. For example, blood pressure cuffs are often smaller and used on the least deformed limb to prevent fractures.
Rodding Surgery
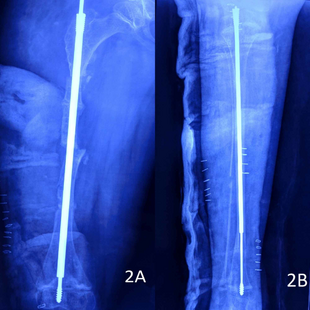
Metal rods can be surgically put into long bones to make them stronger. This procedure was developed by Harold A. Sofield in 1959. It involves carefully breaking the bones, straightening them, and then putting metal rods inside to stabilize them. This treatment helped many people with OI move better. It became standard for severe OI by 1979.
Rodding surgery is often done to help patients with moderate or severe OI walk. About two-thirds of people with severe OI (types III and IV) have had rodding surgery. For type III, surgery is often done earlier because many children cannot walk without it.
The most common rods are IM rods. Some, like the Fassier–Duval IM rod, are "telescoping." This means they can grow as the child grows, reducing the need for more surgeries. The surgery involves breaking the long bones in one or more places, then fixing the rod to keep the bone straight.
While telescoping rods are designed to grow with the child, surgeons sometimes prefer non-telescoping rods for the tibia, which grows less. Rodding surgery has been linked to greater mobility in all types of OI. For type III, it also reduced the number of tibia fractures.
Spinal Surgery
Spinal fusion surgery can correct or prevent scoliosis (curved spine). However, OI bones are fragile, making this surgery more complex. Doctors recommend surgery if the spine's curve is severe (over 50 degrees) after a child has finished growing.
Surgery for basilar invagination (pressure on the brainstem) is only done if it causes serious neurological symptoms. If symptoms appear, surgery is the only way to stop or reverse the problem.
Physical Therapy
Physical therapy is generally recommended, but each person needs a special plan due to OI's variability. Physical therapy helps strengthen muscles, improve movement, and increase flexibility. It must be done gently to avoid fractures. Exercises often include water aerobics, light resistance exercises, and walking (if possible).
Contact sports and activities that stress joints (like jumping) are not recommended. People with limited mobility are encouraged to change positions often. Those in wheelchairs should get out every two hours to exercise and prevent stiffness.
People with moderate to severe OI might find it hard to access pools or gyms. Obesity can also be more common in severe OI, especially after age 20, which can further reduce mobility.
Dental Care
More than half of people with OI also have dentinogenesis imperfecta (DI), a problem with tooth formation. Dental treatment can be challenging due to bone and tooth deformities. Children with OI should see a dentist as soon as their teeth appear. Regular check-ups are important to protect their teeth.
Some OI patients take bisphosphonates. There are concerns about dental complications, but no reports of bisphosphonate-related jaw problems have been found in OI patients.
New Treatments in Development
Monoclonal Antibodies
Scientists are studying monoclonal antibodies for OI. These are special proteins that can target specific things in the body. As of 2021, none are approved for OI.
One antibody, setrusumab, targets sclerostin. Sclerostin normally slows down bone formation. The idea is that reducing sclerostin might help build more bone and strengthen OI bones. Setrusumab is in phase 2/3 trials. Another antibody, romosozumab, is already approved for osteoporosis. Researchers are also studying its use for OI.
Can Osteogenesis Imperfecta be Prevented?
Since OI is a genetic disorder, prevention in the 21st century focuses on preventing affected babies from being born. Genetic counseling helps families understand their options.
Families can consider preimplantation genetic diagnosis (PGD) after in vitro fertilization. This allows them to choose embryos that are not affected by OI. Common OI mutations can be found using exome sequencing or whole genome sequencing. If a pregnancy is already underway, amniocentesis can test the fetus for OI. If the fetus is affected, families can decide whether to continue the pregnancy.
For the most common types of OI, there is a 50% chance of passing the disorder to each child. This is because these types are autosomal dominant. For rare autosomal recessive forms, there is a 25% chance. Genetic testing can help determine the inheritance pattern.
If OI type I is hard to detect at birth, the baby's cord blood can be tested. In more severe cases, OI might be seen on ultrasound during pregnancy. It can be hard to predict how severe OI will be in a child, which is an ethical concern for parents.
If a healthy person has already had a child with OI, there's a small chance future children could also have it due to genetic mosaicism in a parent.
Some people with disabilities and bioethicists criticize prenatal screening for OI. They feel it suggests that lives with OI are "less worth living."
What is the Outlook for People with Osteogenesis Imperfecta?
The prognosis (outlook) for osteogenesis imperfecta depends entirely on its type.
Life Expectancy
For mild type I OI, people usually have a life expectancy similar to the general population. However, for type II, babies rarely live past two years old, often dying in their first weeks. For types III and IV, life expectancy is more complex. Type IV OI is thought to have a near-normal life expectancy. But for type III, it is lower than the general population.
A 2016 study in Denmark found that people with OI had a three times higher risk of death from any cause. This meant a loss of about seven years for females and nine years for males. A 1996 study found that mortality in type III OI is much higher, with many patients dying in their 20s, 30s, and 40s. However, patients who live past age 10 tend to live longer.
Mobility
Adults with mild (type I) OI usually need little adaptive equipment. However, as babies, they might reach motor milestones later than other children.
With adaptive equipment like crutches, motorized wheelchairs, splints, and home changes, many people with moderate to severe OI can be very independent. With treatment and physical therapy, people with type I OI can often walk without help. Those with type III might walk around the house or for exercise. Those with type IV might walk around the house or in the community. However, mobility varies greatly from person to person.
How Common is Osteogenesis Imperfecta?
In the United States, about 1 in 20,000 babies are born with osteogenesis imperfecta. An estimated 20,000 to 50,000 people in the U.S. have OI.
Types I, II, III, and IV are the most common. Type I is the most frequent, about three times more common than type II. The exact numbers for types III and IV are less certain. In a 1989 study in Denmark, type I made up 71% of cases, and type II made up 12%.
Most people inherit OI from a parent. But in many cases, it's a brand new (de novo or "sporadic") mutation in a family. For types of OI that allow survival, type III is most often de novo (85%), followed by type IV (50%) and type I (34%).
Some groups of people might have a higher number of OI cases if they have more carriers of the rare recessive forms of the disease.
History of Osteogenesis Imperfecta
OI has had many names over time, but "osteogenesis imperfecta" has been the most accepted since the late 20th century. Other names include "brittle bone disease" and "fragilitas ossium."
Earliest Records of OI
OI has been found in an ancient Egyptian infant mummified around 1000 BC. The Norse king Ivar the Boneless (around 800 CE) is also thought to have had OI.
Nicolas de Malebranche is often credited with the first description of OI's physical signs in 1688. He described a man whose bones broke easily. However, Malebranche wrongly believed it was caused by the mother watching a public execution.
The first modern scientific studies of OI began in 1788 by Olof Jakob Ekman. He described the condition, including dwarfism, fragile bones, and bowed long bones. In 1831, Edmund Axmann was the first to mention blue sclerae as a sign of OI. Jean Lobstein first described the mild form (now type I) in 1833.
It wasn't until 1912 that hearing loss was recognized as a symptom of OI.
Origin of the Term "Osteogenesis Imperfecta"
Willem Vrolik, a Dutch anatomist, created the term "osteogenesis imperfecta" in 1849. He described the remains of a baby with what is now known as fatal type II OI. Vrolik correctly realized that the condition was a birth defect causing weak bones, not a problem like rickets.
How OI Classification Changed
The way OI is classified has changed as science has advanced. Before modern genetic testing, OI was divided into "congenita" (obvious at birth) and "tarda" (appearing later). The idea that these were different forms of the same disorder was accepted by the 1950s.
The modern system of four types (I, II, III, IV) was introduced in 1979 by David Sillence and others. These types became standard. The newer genetic types (V and higher) have been added as more genetic causes of OI have been found since 2006.
OI in Animals
OI can also affect other animals. In dogs, it's an autosomal recessive condition. Many dog breed organizations offer tests to see if a dog carries the OI gene. Dogs that carry one copy of the gene should only be bred with dogs that don't carry it. Dogs with two copies of the gene should not be bred.
OI has been found naturally in Golden Retrievers, Dachshunds, and Beagles. It has also been identified in zebrafish and mice.
Some of these animal models are used to study human OI. For example, mice with a specific OI gene mutation show symptoms like human type III OI. Other mice models show symptoms like human type I or type II OI. Studying OI in animals can help lead to new treatments for humans.
See also
 In Spanish: Osteogénesis imperfecta para niños
In Spanish: Osteogénesis imperfecta para niños