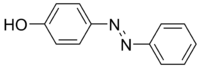Synthetic colorant facts for kids
A colorant is anything that changes the color of a material. Synthetic colorants are special substances made by people in labs or factories. Making and improving these colorants was a huge step for the early chemical industry. In fact, many big chemical companies today, like Bayer AG, started out making dyes in the late 1800s or early 1900s.
Synthetic colorants are very popular for industrial and artistic uses. They often create much brighter colors and last longer than natural dyes and pigments used for thousands of years. Large-scale production of these dyes began almost at the same time in Britain (1857), France (1858), Germany (1858), and Switzerland (1859). The period from the mid-1800s until World War II saw a huge increase in the types and amounts of synthetic colorants made. They quickly became common everywhere, from clothes to food. This happened because of new research labs in the 1870s and a better understanding of how chemicals are built. The dye industry was one of the first times that focused scientific research regularly led to new products.
Contents
- Dyes and Pigments
- History of Colorants
- Important Colorants
- How Colorants Work
Dyes and Pigments
Colorants can be split into two main types: pigments and dyes.
- Dyes usually dissolve in liquid and soak into the material they are coloring. Think of dyeing a T-shirt.
- Pigments don't dissolve. They need something to stick them to a surface, like paint on a wall.
Dyes must be able to stick to the material they color. Chemically, pigments can be organic (from living things or carbon-based) or inorganic (from minerals or non-living things). Dyes are always organic. There's a special type called lake pigments, which are dyes that have been changed to become insoluble, acting like pigments. Historically, both pigments and dyes have been very important, and their markets have always been closely linked.
History of Colorants
People have used colorants since ancient times. Our ancestors used natural materials, mostly from plants and animals, to color their homes and tools. Cave paintings, like those in Altamira or Lascaux, were made 15,000 to 30,000 years ago during the Ice Age. Using pigments for color is one of the oldest human activities. Before modern times, important materials like cotton, silk, wool, leather, and paper were all natural.
The 1800s and 1900s saw a big jump in how much colorant was used and made. This led to many pigments and dyes we still use today. It became possible to create strong acids like sulfuric acid and synthetic sodium carbonate cheaply. This was thanks to new industrial methods, like the Leblanc process, which made these chemicals much more affordable. However, many early colorants are no longer made because they were too expensive or too toxic. Examples include Schweinfurt green and Scheele's Green, which contained arsenic, and Naples yellow, which contained lead.
In the late 1850s, the first modern synthetic dyes appeared, bringing more vibrant colors to Europe. These new dyes were very bright and varied, but they often faded quickly when exposed to sunlight or washing. This problem led to new ways of studying colorants, which in turn helped scientists create more color-fast (longer-lasting) modern colorants. Synthetic colors quickly appeared in dyes, paints, inks, and even food, becoming a big part of everyday life.
Natural Color Sources
In ancient cave paintings, people used natural manganese oxide and charcoal for black. They used iron oxides for yellow, orange, and red. Other natural earth pigments used for a long time include:
- Red: vermilion (from mercury sulfide)
- Yellow: orpiment (from arsenic trisulfide)
- Green: malachite (a copper carbonate)
- Blue: lapis lazuli (a natural ultramarine)
Natural white pigments include chalk and kaolin. Black pigments often came from charcoal and soot.
Early Synthetic Colors
Before the Industrial Revolution, people made various inorganic pigments. Many of these, like Egyptian blue, used toxic chemicals such as arsenic. These toxic pigments were used for cosmetics and painting. In ancient Egypt, blue was seen as a divine color. So, Egyptian Blue became a very important pigment. It was used to show eyes, hair, and decorations on pharaohs. Blue, especially ultramarine made from ground lapis lazuli, remained important for showing holy figures through the Renaissance. Painters in Europe before the Industrial Revolution used ultramarine almost only for the robes of Mary because it was so expensive. Later, Jean-Baptiste Guimet and Christian Gmelin found ways to make it cheaper and more available.
In the early 1700s, the first products of the new color industry were Prussian blue and Naples yellow. The first white pigment made synthetically was white lead (lead carbonate), known since Roman times. Around 1800, more inorganic white pigments were developed, including zinc white (zinc oxide) and antimony white (antimony oxide). Printers and dyers at that time had access to various chemicals like lead acetate, alum, and sulfuric acid. Small workshops grew into much larger factories.
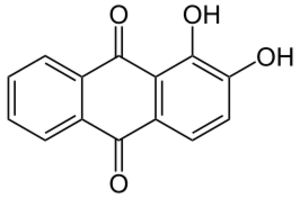
Other inorganic pigments developed in the 1800s included cobalt blue, Scheele's green, and chrome yellow. The availability of strong acids helped with more experiments. This led to the discovery of alizarin and purpurin in 1826.
First "Scientific" Dyes: Aniline Dyes (1858–1870)
In the mid-1800s, the coal tar industry, especially in England, produced many chemicals needed for making organic compounds. For the first eight years after the first successful synthetic dye, Mauveine, British and French companies were the main dye makers. By the late 1860s, German dye factories started to make more dyes and sell more than their competitors. By 1870, German companies made about half of the world's dyes and pigments.
Aniline dyes were made in large amounts because of new ways to create their ingredients. For example, Antoine Béchamp found an easy way to make aniline in 1854. This made the process flow like this: coal tar → nitrobenzene → aniline → dyes. William Henry Perkin, a pioneer in this field, said that this industry was "entirely the outcome of scientific research."
Picric Acid: The First Synthetic Dye
The very first synthetic dye was picric acid. It was made in a lab in 1771 and sold by M. Guinon in Lyon in 1845. It dyed silk yellow, but the color didn't last well, so it wasn't a big commercial success. However, French dyers did buy small amounts of it.
William Perkin's Mauveine
In 1856, 18-year-old William Henry Perkin accidentally discovered a dye he called mauve. He was trying to make quinine (a medicine) in his home lab. Instead, he created a black product. After cleaning it, he had a beautiful mauve dye. Perkin quickly filed a patent in August 1856, and a new dye industry was born! He first called his discovery Tyrian purple, linking it to a very old, expensive dye. Other names included aniline purple and Perkin's mauve. The original mauveine was actually a mix of four main compounds, plus other similar ones.
Perkin started making his dye near London in late 1857. He was the only producer for several months. He even began making the ingredients for his dyes himself, like nitro-benzene, making his operations bigger. By the summer of 1859, a magazine joked that London had "the mauve measles" because the color was so popular.
Fast Growth of the Dye Industry
By the end of 1858, eight companies were already making aniline dyes. By 1861, there were 29 British patents for aniline dyes. By 1864, 68 companies were making dyes. This growth was driven by the textile industry, which wanted new designs using these colorful aniline dyes. Even Perkin's former teacher, August Wilhelm von Hofmann, who had first criticized Perkin for leaving science, later made his own aniline dye, rosaniline.
In 1858, German chemist Johann Peter Griess made a yellow dye by mixing nitrous acid with aniline. It didn't sell well, but it made people even more interested in aniline for colorful compounds. French chemist François-Emmanuel Verguin made fuchsine, a rose-colored dye, by reacting aniline with stannic chloride. This was the first of the triphenylmethane dyes. Further work by Hofmann and the discovery of benzene’s structure (1858) and carbon’s ability to form four bonds (1865) laid the foundation for modern organic chemistry.
By the late 1860s, many companies offered a full range of colors. They were already selling more than many natural dyes. Prices kept falling, and new colors and products regularly appeared. By 1868, there were 52 companies making aniline dyes. Scientists and factory workers across Europe competed to create new dyes. Many soluble acid dyes used for textiles were turned into insoluble pigments by reacting them with salts of calcium, barium, or lead.
Synthetic Alizarin (1868–1873)
The creation of synthetic alizarin opened up a huge market that was previously served by natural dye makers. Alizarin was the first dye whose chemical structure was figured out. Scientists quickly aimed to make it in the lab, and they succeeded by 1868. Other chemicals from natural madder plants were also identified and used by the mid-1800s, like purpurin, which made a delicate lilac color. Like aniline dyes, the ingredients for synthetic alizarin were easily found in coal tar. Germany became the leader in the synthetic alizarin market.
Azo-Dyes from Coupling Reactions (1878–1885)
In 1858, Peter Griess discovered a new group of compounds called Azo dyes. Later, a new type of azo dye, made by "coupling" reactions, became available. These new azo dyes were easy to make and came in a huge variety of incredibly bright colors, depending on the chemicals used. Several chemists sold azo dyes and tried to keep their methods secret. However, Hofmann figured out their chemical structures and shared his findings.
This led to another rapid expansion, especially in Germany. Between 1877 and 1887, 130 German patents were filed for azo dyes, and 105 new dyes reached the market. This also changed how chemical companies worked with customers. German dye companies developed their own marketing and distribution teams that worked closely with their research departments. By World War I, azo dyes were the most common type of dye sold. In 1885, a red azo dye called Para red became the first organic pigment that didn't dissolve in water and didn't contain acidic or basic parts.
New Dyes and Bigger Markets (1900–1913)
The 1900s brought even more growth in chemical production. New pigments like cadmium selenide and bismuth vanadate were created. High-purity titanium dioxide and zinc oxide were made on a large scale for the first time, introducing new synthetic white pigments. The first insoluble organic pigments, the red naphthols, were also produced and sold.
The quality of new dyes also improved. Chemist Rene Bohn developed a brilliant blue dye called Indanthrone blue in 1901, which had excellent color fastness (it lasted a long time). BASF, the largest maker of these dyes, sold it as Indanthrene blue RS, along with the synthetic indigo they started selling in 1897.
Allegedly, James Morton, a leader in England's textile industry, was upset when he saw some tapestries he made with aniline dyes had already faded. He started testing dye samples by exposing them to the sun to check how well they resisted light. He then hired a chemist to create dyes that were more stable in sunlight. He marketed these dyes as fast dyes or sundour, which means "hard to move" in Scots.
By this time, synthetic dyes were made in many countries, including Britain, Germany, France, the US, Switzerland, and Italy. Chemical plants also grew much larger. For example, the Bayer company in 1907 had a reactor to make azo dye with a capacity of 20,000 liters. From 1900 until World War I, German companies controlled about 75% of the dye market. World War I disrupted this, and the chemical industry in the United States grew quickly, though Germany remained a major player.
World War I and American Dyes (1913–1930)
Before 1914, the US dye market mostly relied on German imports. There were only a few small American companies. But with World War I, German dye factories had to switch to making explosives, and British blockades cut off German shipping. Dye prices quickly went up, and US companies built factories to meet the demand. Big American pharmaceutical companies like Dow and DuPont started making dyes and were very successful with simple sulfur and vat dyes. Dow Chemical developed a way to make synthetic indigo in 1915. American industry and universities worked together to figure out German chemical production secrets. After the war, some American factories that made weapons switched to making dyes, realizing that if Germany could do the reverse during the war, they could too.
Artistic Use of Colorants
Synthetic colorants quickly became popular with artists as well as in industry. The Impressionist painters, in particular, were famous for using them early on. Critics sometimes compared the Impressionists' blues to laundry tubs or chemical waste in the Seine river, because the colors were so new and bright. One critic even said Edgar Degas had an "obsession with chemistry," describing his studio like a lab. Interestingly, Degas was known to talk with chemist Marcellin Berthelot, who is considered the father of organic synthetic chemistry in France. Pierre-Auguste Renoir’s later paintings used a lot of alizarin crimson. He also used cobalt blue or a mix of ultramarine and cobalt blue, which are synthetic pigments. New pigments and dyes weren't just for European artists; even Japanese printmakers were using dyes like rosaniline as early as 1863.
Important Colorants
Prussian Blue
Prussian blue, also known as Berlin blue, Paris Blue, or Turnbull's Blue, is an inorganic pigment. It's made in large amounts for both art and textiles. Its chemical formula is FeIII~4~[FeII~(CN)~6~]~3~. Prussian blue has been around since the early 1700s and is still a popular art pigment. Studies of Prussian Blue led to discoveries about hydrogen cyanide. It is also used as an antidote for heavy metal poisoning. It's famous for being used to color the uniforms of the Prussian Army in the 1700s.
Mauveine
Mauveine was discovered by Henry Perkin when he was trying to make quinine. He added aniline, a simpler chemical, and it created a black product. After cleaning it, Perkin had a mauve dye. Perkin filed his patent in August 1856, and this started a new dye industry. He first called his discovery Tyrian purple, referring to an ancient, very expensive dye. Other names include aniline purple and Perkin's mauve. The original mauveine wasn't just one molecule; it was mainly a mix of four compounds: mauveine A, mauveine B, mauveine C, and mauveine B2, along with other similar ones.
Synthetic Alizarin
Natural Alizarin was the first colorant whose chemical structure was fully understood. This made it one of the first targets for scientists to try and make in the lab. The first successful way to make alizarin was patented by Carl Gräbe and Carl Theodore Liebermann in 1868. A second, much cheaper, way to make it was developed in 1869 by Graebe, Liebermann, and Heinrich Caro. Perkin submitted his own patent for a very similar process just one day later and was granted the patent in England.
How Colorants Work
Colorants work by absorbing certain wavelengths of light in the visible spectrum. A pigment or dye molecule absorbs different colors of light depending on its atomic structure. This means that when light hits a colorant, some colors are absorbed, and others are reflected or transmitted, which is what we see. The physical shape, size, and amount of dyes and pigments can also greatly change the color we see. Pigments are especially affected by their physical properties.
Most modern synthetic dye molecules have two main parts. The first part is a ring of carbon atoms, often a benzene ring or a system of them. The second part is a chromophore, which is a system of double bonds. This is the part that absorbs or reflects color when exposed to visible light. Other parts of the colorant molecules can change how bright the color is, its exact shade, how well it dissolves, and how well it sticks to a material.
Dyes and pigments can be sorted by how they are made or their chemical properties. For example, British chemist Edward Chambers Nicholson showed that pure aniline didn't make dye. Hofmann showed that another chemical called toluidine had to be present to make these dyes. Aniline dyes, including mauve, are made from aniline that contains some toluidine. Dyes can also be classified by their chemical formulas, like azo-dyes, which have a special azo group.