Leblanc process facts for kids
| Process type | Chemical |
|---|---|
| Industrial sector(s) | Chlor-alkali industry |
| Feedstock | sodium chloride, sulfuric acid, coal, calcium carbonate |
| Product(s) | soda ash, hydrochloric acid, calcium sulfide, carbon dioxide |
| Inventor | Nicolas Leblanc |
| Year of invention | 1791 |
| Developer(s) | William Losh, James Muspratt, Charles Tennant |
The Leblanc process was an early way to make soda ash (sodium carbonate). It was used a lot during the 1800s. This process is named after its inventor, Nicolas Leblanc. It had two main steps. First, it made sodium sulfate from regular salt (sodium chloride). Then, it mixed the sodium sulfate with coal and calcium carbonate to create soda ash. Over time, a newer and better method called the Solvay process replaced the Leblanc process.
Contents
Why We Needed Soda Ash
Soda ash (sodium carbonate) and potash (potassium carbonate) are both called alkali. These chemicals are super important for making things like glass, textiles (cloth), soap, and paper.
For a long time, people in Europe got alkali from burning wood to make ashes. But by the 1200s, too many trees had been cut down. This made it too expensive to get alkali this way. So, Europe had to buy alkali from other places. Potash came from North America, Scandinavia, and Russia, where there were still huge forests. Soda ash came from Spain and the Canary Islands. There, it was made from the ashes of special plants called glasswort. In Egypt, people found natural soda ash called natron in dry lakebeds. In Britain, the only local source was from kelp, a type of seaweed found on the coasts of Scotland and Ireland.
In 1783, the King of France, Louis XVI of France, and the French Academy of Sciences offered a prize. They wanted someone to find a way to make alkali from sea salt (sodium chloride). In 1791, a doctor named Nicolas Leblanc found a solution and got a patent for it. That same year, he built the first Leblanc factory near Paris. It started making 320 tons of soda every year. But because of the French Revolution, he never received his prize money.
How the Leblanc Process Worked
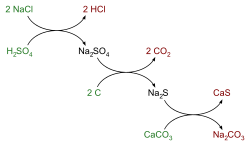
The Leblanc process had two main chemical steps.
Step 1: Making Salt Cake
First, regular salt (sodium chloride) was mixed with sulfuric acid. This part of the process is called the Mannheim process. This reaction created sodium sulfate, which was also known as salt cake. It also produced hydrogen chloride gas.
- Salt + Sulfuric Acid → Sodium Sulfate + Hydrogen Chloride Gas
- 2 NaCl + H2SO4 → Na2SO4 + 2 HCl
A Swedish scientist named Carl Wilhelm Scheele had discovered this reaction in 1772.
Step 2: Making Soda Ash
Leblanc's big idea was the second step. He took the salt cake and mixed it with crushed limestone (calcium carbonate). Then, he heated this mixture with coal. The coal helped to change the chemicals.
First, the coal (which is a source of carbon) reacted with the sodium sulfate. This turned the sulfate into a sulfide:
- Sodium Sulfate + Coal → Sodium Sulfide + Carbon Dioxide
- Na2SO4 + 2 C → Na2S + 2 CO2
Next, the sodium sulfide reacted with the limestone. This created the soda ash (sodium carbonate) and calcium sulfide. The mixture of these two was called black ash.
- Sodium Sulfide + Limestone → Soda Ash + Calcium Sulfide
- Na2S + CaCO3 → Na2CO3 + CaS
To get the pure soda ash, workers added water to the black ash. The soda ash dissolved in the water. Then, they evaporated the water, leaving behind solid sodium carbonate. This process of dissolving and separating was called lixiviation.
How the Process Was Done
To start, salt was mixed with strong sulfuric acid and heated gently. The hydrogen chloride gas would bubble away. At first, this gas was just let out into the air. Later, factories used special towers to absorb it. What was left was a melted mass. This mass was heated more directly to remove almost all the remaining salt.
The coal used in the next step needed to have very little nitrogen. This was important to stop harmful cyanide from forming. The limestone or chalk used also needed to be pure. For the second step, the mixture usually had 2 parts salt cake, 2 parts limestone, and 1 part coal by weight. This mixture was heated in a special oven called a reverberatory furnace at about 1000 °C (1832 °F). Sometimes, these ovens even rotated!
The black ash that came out of the oven had to be washed with water right away. This stopped the sulfides from changing back into sulfates. To get the most out of the black ash, the washing was done in several steps, like a waterfall. Fresh water was used on the black ash that had already been washed a bit. The water from that wash was then used on earlier batches of black ash, and so on.
Finally, carbon dioxide gas was blown through the liquid. This removed any dissolved calcium and other unwanted bits. It also turned any leftover sulfide into hydrogen sulfide gas, which was carried away. The liquid was then separated and heated to evaporate the water, using heat from the oven. The resulting soda ash was then dissolved again in hot water. Any solids that didn't dissolve were removed. As the solution cooled, almost pure soda ash crystals formed.
The Leblanc Process in History
Leblanc built his first factory in 1791 in St. Denis, France. But during the French Revolution, the government took over his factory in 1794. They also shared his secret process with everyone. Napoleon I gave the factory back to Leblanc in 1801. However, Leblanc didn't have enough money to fix it up and compete with other soda factories that had started. He died in 1806.
By the early 1800s, French factories were making 10,000 to 15,000 tons of soda ash each year. But it was in Britain where the Leblanc process really took off. The first British Leblanc factory was built in 1816. However, high taxes on salt in Britain made it hard for these factories to make money. This changed in 1824 when the salt tax was removed.
After that, the British soda industry grew very fast. Big factories were built by people like James Muspratt in Liverpool and Charles Tennant near Glasgow. Muspratt's factory in Liverpool was in a great spot. It was close to salt mines, coal fields, and limestone quarries. By 1852, Britain was making 140,000 tons of soda each year. France was making 45,000 tons. By the 1870s, Britain's soda production of 200,000 tons each year was more than all other countries combined!
Environmental Impact
Leblanc process factories caused a lot of harm to the environment nearby. When they made salt cake, they released hydrogen chloride gas. In the early 1800s, nobody knew what to do with this gas, so it was just let out into the air. This gas was very bad for plants and animals.
The process also created a lot of smelly, solid waste. For every 8 tons of soda ash made, the process produced 5.5 tons of hydrogen chloride gas and 7 tons of calcium sulfide waste. This solid waste, called galligu, had no use. It was piled up in huge heaps or spread on fields near the factories. When it rained, this waste released hydrogen sulfide, a toxic gas that smells like rotten eggs.
Because of the bad fumes, Leblanc factories faced many lawsuits and new laws. People complained that the gas from these factories hurt plants, animals, and even people's health.
In 1863, the British Parliament passed the Alkali Act 1863. This was one of the first modern laws about air pollution. It said that factories could only release a very small amount (no more than 5%) of the hydrogen chloride gas into the air. To follow this law, factories started passing the gas through towers filled with charcoal. Water flowing through the towers absorbed the gas, turning it into hydrochloric acid solution. But then, chemical factories often dumped this acid into nearby rivers or lakes, which killed fish and other water life.
Working conditions for the people in Leblanc factories were also very tough. The work involved handling hot, harmful chemicals. Workers sometimes wore cloths over their mouths and noses to avoid breathing in dust and fumes. Later, machines helped make the work safer and the products more consistent.
By the 1880s, new ways were found to turn the hydrochloric acid into chlorine gas. This chlorine was used to make bleaching powder. Ways were also found to get the sulfur back from the calcium sulfide waste. Even with these improvements, the Leblanc process was still more wasteful and polluting than the newer Solvay process.
Why the Leblanc Process Was Replaced
In 1861, a Belgian chemist named Ernest Solvay created a better way to make soda ash. His Solvay process used salt and limestone, but it also used ammonia. The only waste product from the Solvay process was calcium chloride, which was much less harmful. This new method was cheaper and caused less pollution than the Leblanc process.
From the late 1870s, Solvay factories in Europe started to compete strongly with the British Leblanc factories. A Solvay factory opened in Britain in 1874, making the competition even tougher. Leblanc factories couldn't compete with the cheaper Solvay soda ash. Their main profit then came from making chlorine and bleaching powder, which were once just unwanted by-products.
But then, new ways of making chlorine using electricity were invented. This took away another source of profit for the Leblanc factories. By 1900, 90% of the world's soda was made using the Solvay method. In North America, people also started mining a natural mineral called trona to get soda ash. The last Leblanc soda ash factory closed in the early 1920s.
However, the Solvay process doesn't work well for making potassium carbonate. So, the Leblanc process was still used a bit longer for that specific chemical.
Special Habitats
Interestingly, the waste from the Leblanc process has created a very rare type of habitat in the UK. The waste breaks down into calcium carbonate, which makes the soil rich in lime. This creates a perfect home for plants that love lime-rich soils, called calcicoles. Only four such sites have survived into the new millennium. Three of them are protected as local nature reserves. The largest one, at Nob End near Bolton, is a special protected area. It's known for its rare orchids and other lime-loving plants, which are very unusual in an area that usually has acidic soils. This "alkaline island" even has a small "acid island" within it, where acidic boiler waste was dumped. This area now has plants like heather, which prefer acid soils.
|
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |