History of penicillin facts for kids
The story of penicillin is about how people found and developed the first widely used antibiotics. Antibiotics are special medicines that fight off harmful bacteria and stop infections. Penicillin was discovered from a type of mould called Penicillium. It became a very important medicine in 1942.
Many old cultures used moulds to treat infections. Over the centuries, people noticed that moulds could stop bacteria from growing. In 1928, a Scottish doctor named Alexander Fleming was working in London. He was the first to prove that a Penicillium mould made a substance that killed bacteria. He called this substance "penicillin." The mould was a type of Penicillium notatum (now called Penicillium rubens). It accidentally grew in his lab.
In 1939, a team of scientists at the University of Oxford started studying penicillin. This team was led by Howard Florey and included Ernst Chain. They figured out how to grow the mould and get pure penicillin from it. They tested it on animals to make sure it was safe and worked well. Then they did tests on people. During World War II, penicillin became very important. It saved thousands of lives for the Allied forces. In 1945, Alexander Fleming, Howard Florey, and Ernst Chain won the Nobel Prize in Physiology or Medicine for their work.
After the war, penicillin became available everywhere. Dorothy Hodgkin figured out its exact chemical structure. She won the Nobel Prize in Chemistry in 1964 for this. Her discovery helped scientists create new types of penicillin. These new penicillins were stronger and could fight more kinds of bacteria. Even today, growing mould is the main way to make penicillin.
Contents
- Early Discoveries of Mould's Power
- How Penicillin Was Discovered
- Getting Penicillin Ready for Use
- Testing Penicillin on People
- Making Penicillin in Large Amounts
- Penicillin in the War Zone
- The Nobel Prize for Penicillin
- New Types of Penicillin
- Bacteria Fighting Back: Antibiotic Resistance
- Penicillin in Farming
Early Discoveries of Mould's Power
Many ancient groups, like those in Australia, China, Egypt, Greece, and India, found out that certain fungi and plants could help treat infections. These natural treatments often worked because many organisms, including moulds, naturally make antibiotics. However, people back then could not find or separate the active parts of these organisms.
In 1640 England, a botanist named John Parkinson wrote about using moulds to treat infections. In Poland during the 1600s, people used wet bread mixed with spider webs to treat wounds. Spider webs often had mould spores on them.
In the late 1800s, scientists started to notice this effect more closely. In 1871, Sir John Scott Burdon-Sanderson reported that mould on a culture fluid stopped bacteria from growing. Joseph Lister, a famous English surgeon, also saw that mould in urine samples stopped bacteria. In 1875, John Tyndall showed the Royal Society how Penicillium fungus could kill bacteria.
In 1877, French scientists Louis Pasteur and Jules Francois Joubert saw that other bacteria could stop anthrax bacteria from growing. They called this effect antibiosis, meaning "against life." This term later became "antibiotic" in 1947.
In 1895, an Italian doctor, Vincenzo Tiberio, found that moulds in a water well had substances that killed bacteria. Two years later, Ernest Duchesne in France found that a P. glaucum mould could heal infected guinea pigs. He published his findings in 1897. He didn't know exactly what in the mould helped, but he saw it protected the animals.
How Penicillin Was Discovered
In 1928, Alexander Fleming was studying bacteria called Staphylococcus aureus at St Mary's Hospital, London. He went on vacation in August. Before leaving, he left some culture plates with S. aureus on his lab bench. When he came back on September 3, he looked at the plates. He found one with an open lid that had a blue-green mould growing on it.
Around the mould, the bacteria had not grown, but farther away, they grew normally. This meant the mould was killing the bacteria. Fleming took a sample of the mould to identify it. He then grew the mould again and repeated the experiment. He saw the same bacteria-killing results.
Fleming realized the mould was releasing a substance that stopped bacteria from growing. He tested it on different bacteria. He found it could kill certain Gram-positive bacteria like Staphylococcus and streptococcus. But it did not affect others, like typhoid bacteria. On March 7, 1929, he named this substance "penicillin." He chose the name because it came from the Penicillium mould.
Fleming thought his mould was Penicillium chrysogenum. Later, in 2011, scientists found that Fleming's mould was actually Penicillium rubens.
For many years, people wondered how the mould got into Fleming's lab. Fleming thought the spores came through his window. But his co-workers said the window was always shut. Most people now agree the mould came from a lab downstairs. Spores likely drifted in through open doors.
It was lucky that the temperature in the lab was just right. First, it was cool enough for the mould to grow. Then, it got warmer, which was good for the bacteria. If Fleming had put the cultures in an incubator, this amazing discovery might not have happened.
Fleming was a bacteriologist, not a chemist. He tried to get others to help him isolate penicillin, but they couldn't. Because of this, Fleming stopped his chemical research on penicillin in 1929. He published his findings in a journal, but it didn't get much attention at first.
Getting Penicillin Ready for Use
In 1939, Ernst Chain found Fleming's old paper about penicillin. He suggested to his boss, Howard Florey, that they should study substances made by tiny living things that fight bacteria. Florey led a team at the Sir William Dunn School of Pathology at the University of Oxford. They started working on penicillin. At first, they didn't think it would be a useful medicine.
The Oxford team's first step was to get a sample of the penicillin mould. This was easy because a previous researcher had kept a sample. Next, they needed to grow enough mould to get penicillin for experiments. They grew the mould on a liquid surface. After a few days, it formed a yellow skin with green spores. The liquid underneath turned yellow and contained penicillin.
The mould needed air to grow, so they used containers with large flat surfaces. They even used bedpans at first! Later, they had special ceramic containers made that could be stacked. They tried adding different things to the mould's food to make it produce more penicillin. Adding brewing yeast didn't increase the amount, but it made the mould grow faster. They also learned that some airborne bacteria could destroy penicillin. So, they had to grow the mould in very clean conditions.
The next step was to get the penicillin out of the liquid. They filtered the liquid to remove the mould. Then they made the liquid more acidic and cooled it down. Penicillin is only stable in a certain pH range. Keeping it cold helped stop it from breaking down. They used a solvent called amyl acetate to pull the penicillin out of the liquid.
Then came the challenge of getting the penicillin out of the solvent. A scientist named Norman Heatley had an idea. If penicillin moved from water to solvent when acidic, maybe it would move back to water if it was alkaline. He tried adding Sodium hydroxide, and it worked! This was called "reverse extraction."
The final problem was how to get the penicillin out of the water without destroying it. Boiling or evaporating would ruin it. Chain thought of freeze drying, a new technique. This removed the water, leaving a dry, brown powder.
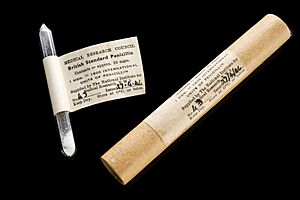
Heatley also created a test to measure penicillin's strength. They put the penicillin liquid in small cylinders on plates with bacteria. After some time, a clear ring formed around the cylinder where bacteria couldn't grow. The size of the ring showed how strong the penicillin was. They found that even a tiny amount of impure penicillin could kill bacteria. It was much stronger than other medicines available then.
In March 1942, the Oxford team announced they could make a very pure form of penicillin. They also figured out its chemical formula.
Testing Penicillin on People
Howard Florey's team at Oxford showed that penicillin extract killed many different bacteria. They found it stopped gonococcus, meningococcus, streptococcus, staphylococcus, and even gangrene bacteria. They noticed that penicillin didn't kill bacteria directly. Instead, it stopped them from dividing and multiplying. They also found it didn't harm white blood cells, which are part of the body's natural defense. Unlike other medicines, penicillin was not destroyed by pus.
By March 1940, the Oxford team had enough impure penicillin to test if it was harmful. They tested it on rats, mice, rabbits, and cats. They gave penicillin in different ways. Their tests showed that penicillin was destroyed in the stomach. But injections worked well. They found no signs of harm in any of their animals. (It's lucky they didn't test on guinea pigs, as penicillin is toxic to them!)
On May 25, 1940, Florey injected eight mice with a strong type of streptococcus. Four of them also got penicillin. By the next morning, all the untreated mice were dead. All the treated ones were still alive. Florey called this result "a miracle." They repeated the experiment many times with similar success. They found penicillin also worked against staphylococcus and gas gangrene. But Florey reminded his team that humans are much bigger than mice.
The Oxford team published their results in a famous medical journal, The Lancet, on August 24, 1940. They said penicillin was effective against infections. But the article didn't get much attention at first. Alexander Fleming visited them in September 1940. He saw their work but didn't say much.
In the United States, other scientists read the Lancet article. They started their own penicillin research. On October 15, 1940, two patients in New York City became the first people in the US to receive penicillin. Their work got a lot of public interest.
Back in Oxford, they started human trials. On January 17, 1941, a woman with cancer received penicillin to test for side effects. Her temperature went up a little, but she had no other bad reactions.
The first patient treated for an infection was Albert Alexander, a policeman with a severe facial infection. On February 12, he received penicillin doses. Within a day, he started to get better. His fever dropped, and his wounds improved. However, the researchers didn't have enough penicillin to fully cure him. He sadly died on March 15.
Because of this, Florey decided to focus on treating children. They needed smaller amounts of penicillin. Several children were successfully treated. One was a 15-year-old boy with a hip infection who recovered completely.
By January 1942, commercial companies started producing penicillin. Florey's wife, Ethel Florey, led more clinical trials. They published their results in The Lancet in March 1943. They reported treating 187 cases of severe infections with penicillin. This evidence convinced the British government to start mass production of penicillin.
Making Penicillin in Large Amounts
The Oxford team knew they couldn't make enough penicillin in their lab for everyone. They tried to get the British government and companies to help, but at first, no one was very interested.
In April 1941, Howard Florey and Norman Heatley went to the United States. They hoped to find a way to make penicillin in large amounts there. They even smeared mould on their coat pockets so they wouldn't lose the samples!
In the US, Florey and Heatley met with scientists at the United States Department of Agriculture (USDA) lab in Peoria, Illinois. They talked about using large tanks for fermentation, which might be the key to mass production.
A scientist named Andrew J. Moyer worked with Heatley. Moyer found that adding corn steep liquor (a byproduct from corn processing) to the mould's food made the penicillin yield ten times higher!
In March 1942, a woman named Anne Sheafe Miller was dying from a severe infection. Her doctor asked for penicillin. Heatley was working with a company called Merck. They sent 5.5 grams of penicillin by air. Within 48 hours, her high fever dropped, and she started eating again. She made a full recovery and lived until 1999.
Until May 1943, most penicillin was made in shallow dishes. But a USDA scientist, Kenneth Bryan Raper, tried growing mould in large vats. This was called "deep submergence production." At first, it didn't make much penicillin. But then, they found a better mould strain from a cantaloupe in a Peoria fruit market! This mould, called NRRL 1951, produced much more penicillin.
Scientists then used X-rays and ultraviolet light to create even better mutant strains of the mould. One strain, Q-176, produced twice as much penicillin. Adding Phenylacetic acid helped it make the most powerful type of penicillin, called penicillin G. This strain could produce a lot of penicillin per litre.
Pfizer, a company that made citric acid, was good at deep submergence techniques. They put all their effort into making penicillin this way. They invested a lot of money. They solved problems like foaming during the process by adding an anti-foaming agent.
Pfizer opened a small production plant in August 1943. One tank soon made half of the company's penicillin. Then, they bought an ice plant and turned it into the first large deep-submergence penicillin factory. It opened in March 1944.
Penicillin Production Around the World
Australia's Contribution
In mid-1943, Australia decided to make its own penicillin. A production plant was set up in Parkville, Victoria. The first Australian-made penicillin reached soldiers in New Guinea by December 1943. By 1944, Australia was making enough penicillin for civilians too.
Canada's Role
In August 1943, the Canadian government asked the Connaught Laboratories to start mass production. They bought a building and turned it into a penicillin factory. At first, they grew penicillin in 200,000 bottles. In November 1945, they switched to the deep submergence method.
Europe's Efforts
News of penicillin reached Germany in 1942. Research was done in different places. Some penicillin captured by German forces reached Germany in 1943. Scientists started experimenting with moulds. Hitler's doctor even treated him with penicillin after an assassination attempt in 1944.
After the war, penicillin factories were built in many European countries. Canada helped with this through the United Nations Relief and Rehabilitation Administration (UNRRA).
Japan's Production
News about penicillin reached Japan in late 1943. By February 1944, penicillin production was underway. By mid-May, a research team had found many mould strains that produced antibiotics. Small production plants opened in Japan in late 1944 and early 1945. By 1948, Japan was making enough penicillin for itself.
United Kingdom's Factories
In the UK, companies like Kemball, Bishop & Co. and Imperial Chemical Industries (ICI) started making penicillin in 1941 and 1942. Wartime conditions, like German bombing, made it hard. But production grew. ICI's plants produced millions of units per week.
Glaxo Laboratories also opened several plants. They switched to using corn steep liquor and better mould strains. By 1943, Glaxo made most of the UK's penicillin. In 1946, the UK switched completely to the deep submergence method. Penicillin production in the UK increased hugely from 1943 to 1946.
United States' Massive Output
In July 1943, the US government put penicillin under a wartime system. All supplies went to the armed forces and public health. Penicillin production in the US grew from 800 million units in early 1943 to 20 billion units in the second half. The US government built six production plants. Private companies built sixteen more.
US penicillin production soared. By June 1944, Pfizer alone was making 70 billion units per month. The price of penicillin dropped sharply as more was produced.
At first, penicillin was only given for very serious cases that other treatments couldn't help. Military needs took 85% of the production in 1944. By 1945, civilian demand was very high. Penicillin became available for sale to the public by the end of 1945. The United States was exporting 200 billion units a month.
Penicillin in the War Zone
In 1943, the Medical Research Council decided to test penicillin in real-life situations. Howard Florey went to North Africa, where a war was happening. He was joined by Hugh Cairns, a British Army brigadier and surgeon. Cairns brought a large supply of penicillin.
Over two months, Florey and Cairns treated more than a hundred soldiers. Many had old wounds that hadn't healed. They wrote a long report with recommendations for how doctors should use penicillin.
Florey believed that many infections came from hospitals, not just the battlefield. He suggested new ways to treat wounds. He said wounds should be cleaned and closed quickly. This was a new idea, as normally this could cause infections like gas gangrene. But he trusted penicillin to prevent it. His ideas were put into action.
During the war in Western Europe (1944–1945), penicillin was used widely. It treated infected wounds and prevented new infections. In World War I, gas gangrene killed many soldiers. With penicillin, this disease almost disappeared. Recovery rates for broken bones and burns improved dramatically.
The Nobel Prize for Penicillin
When news of penicillin's healing power became public in 1942, Alexander Fleming enjoyed the attention. But Howard Florey did not. He worried it would create demand for a medicine they didn't have enough of yet. He told his team not to talk to the press.
This led to confusion. Many stories gave Fleming all the credit for penicillin's development. Fleming and his hospital did little to correct these stories. People wanted to hear the simple story of one scientist making a lucky discovery. But the truth was, developing penicillin into a usable drug took a huge team effort.

In 1943, the Nobel committee received a nomination for Fleming and Florey for the Nobel Prize in Physiology or Medicine. In 1945, many nominations came in for Florey, Fleming, or both. Finally, the committee decided to divide the prize equally among three people.
On October 25, 1945, it was announced that Alexander Fleming, Howard Florey, and Ernst Chain would share the 1945 Nobel Prize. They won "for the discovery of penicillin and its curative effect in various infectious diseases."
Later, in 1964, Dorothy Hodgkin received the Nobel Prize in Chemistry. She won for figuring out the exact structure of important biological substances, including penicillin.
New Types of Penicillin
The first penicillin only worked against certain diseases. Scientists wanted to find new types that could treat more infections. They found a way to get the main part of penicillin, called 6-APA. This allowed them to make "semisynthetic" penicillins. These new versions were better than the original.
Ampicillin was developed in 1961. It was the first semisynthetic penicillin that could be taken by mouth. It worked against both Gram-negative and Gram-positive bacteria. The original penicillin only worked against Gram-positive ones.
Other new penicillins were developed that could fight bacteria that had become resistant to the original penicillin. These included flucloxacillin and methicillin. Scientists also made penicillins that were good at fighting specific Gram-negative bacteria.
The core structure of penicillin, called the β-lactam ring, was so useful that other antibiotics, like cephalosporins, still use it. Amoxicillin, developed in 1970, became one of the most commonly used penicillins worldwide.
Bacteria Fighting Back: Antibiotic Resistance
In 1940, Ernst Chain and Edward Abraham found the first sign of antibiotic resistance to penicillin. They found a type of E. coli bacteria that made an enzyme called penicillinase. This enzyme could break down penicillin, making it useless.
In his Nobel lecture, Alexander Fleming warned about bacteria becoming resistant. He said that if people could buy penicillin easily and didn't take enough, their bacteria might become resistant.
At that time, only poisons needed a doctor's prescription. Self-treating with penicillin was possible. So, laws were passed in the UK in 1947 and the US in 1951 to require a prescription for antibiotics.
However, in many parts of the world, antibiotics spread faster than medical knowledge. People often used them incorrectly. Even in 1999, a study in the UK found that many people wrongly believed antibiotics could cure colds and flu. This led to people demanding antibiotics for things they didn't need them for. Doctors, sometimes overworked, would often prescribe them.
By 1942, some types of Staphylococcus aureus bacteria had become very resistant to penicillin. By the 1960s, many strains were resistant. In 1946, a bacteriologist named Mary Barber studied penicillin resistance. She found that in 1946, most bacterial infections could be treated with penicillin. But two years later, far fewer could.
The problem was made worse by poor hygiene in hospitals and by doctors giving antibiotics when they weren't truly needed. In 1965, the first case of penicillin resistance in Streptococcus pneumoniae was reported. Since then, many other types of bacteria have developed resistance.
Penicillin in Farming
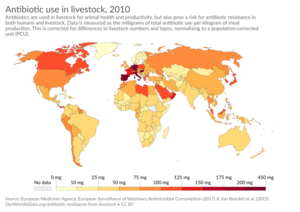
In the late 1940s, research showed that adding penicillin to chicken feed made them gain weight faster. The reasons for this are still debated today. Later studies found that penicillin in animal feed also helped animals use their food more efficiently. It also promoted more even growth and helped control diseases.
After the Food and Drug Administration approved penicillin for poultry and livestock feed in 1951, drug companies made much more of it. By 1954, a lot of the antibiotics made in the US went into animal feed. By the 1990s, half of all antibiotics produced in the US were for livestock.
In the mid-1950s, there were reports that milk wasn't curdling to make cheese. The FDA found that the milk had penicillin in it. The penicillin was killing the good bacteria needed for cheesemaking. By 1963, the World Health Organization reported high levels of penicillin in milk worldwide. People allergic to penicillin could even have a reaction from drinking milk.
In the UK, a committee was set up in 1960 to look into using antibiotics in animal feed. In 1962, they said that rules on using antibiotics in animals should be relaxed. They thought the benefits were great. They also believed that even if bacteria became resistant, new antibiotics would be developed. They didn't think animal resistance affected human health.
However, new research soon showed that bacteria could not only pass on genes for antibiotic resistance but also share them with each other. In 1967, a type of E. coli that was resistant to many antibiotics killed fifteen children in the UK. Because of this, the use of antibiotics in animals for non-medical reasons was banned in the UK in 1971. Many other European countries soon followed.
When Sweden joined the European Union (EU) in 1995, they had already banned antibiotics for growth promotion in animals for ten years. This led to the EU recommending strong limits on antibiotic use. In December 1996, the European Parliament voted to ban the use of antibiotics to promote growth in animals. The EU went even further, suggesting wide restrictions on antibiotic use.