Corundum facts for kids
Quick facts for kids Corundum |
|
|---|---|
 |
|
| General | |
| Category | Oxide mineral – Hematite group |
| Formula (repeating unit) |
Al2O3 |
| Strunz classification | 4.CB.05 |
| Dana classification | 4.3.1.1 |
| Crystal symmetry | R3c (No. 167) |
| Unit cell | a = 4.75 Å, c = 12.982 Å; Z = 6 |
| Identification | |
| Color | Colorless, gray, golden-brown, brown; purple, pink to red, orange, yellow, green, blue, violet; may be color zoned, asteriated mainly grey and brown |
| Crystal habit | Steep bipyramidal, tabular, prismatic, rhombohedral crystals, massive or granular |
| Crystal system | Trigonal |
| Twinning | Polysynthetic twinning common |
| Cleavage | None – parting in 3 directions |
| Fracture | Conchoidal to uneven |
| Tenacity | Brittle |
| Mohs scale hardness | 9 (defining mineral) |
| Luster | Adamantine to vitreous |
| Streak | Colorless |
| Diaphaneity | Transparent, translucent to opaque |
| Specific gravity | 3.95–4.10 |
| Optical properties | Uniaxial (−) |
| Refractive index | nω = 1.767–1.772 nε = 1.759–1.763 |
| Pleochroism | None |
| Melting point | 2,044 °C (3,711 °F) |
| Fusibility | Infusible |
| Solubility | Insoluble |
| Alters to | May alter to mica on surfaces causing a decrease in hardness |
| Other characteristics | May fluoresce or phosphoresce under UV light |
| Major varieties | |
| Sapphire | Any color except red |
| Ruby | Red |
| Emery | Black granular corundum intimately mixed with magnetite, hematite, or hercynite |
Corundum is a very special type of crystal that is made mostly of aluminium oxide (that's aluminum and oxygen, written as Al
2O
3). It often has tiny amounts of other metals like iron, titanium, vanadium, and chromium mixed in. Corundum is a rock-forming mineral, which means it's one of the building blocks of Earth's rocks.
Naturally, corundum is clear, but it can come in many different colors! These colors happen because of those tiny bits of other metals (called transition metal impurities) inside its crystal structure.
Corundum is famous for two main types of gems:
- Ruby: These are red because they contain chromium.
- Sapphire: These can be almost any other color, depending on which metal impurities are present. For example, blue sapphires often have iron and titanium. There's even a rare pink-orange sapphire called padparadscha.
The name "corundum" comes from an old word, kurundam, used in the Tamil and Dravidian languages, which referred to ruby and sapphire. It also appears in Sanskrit as kuruvinda.
Contents
Why is Corundum So Hard?
Corundum is incredibly hard! On the Mohs scale of mineral hardness, pure corundum is a 9. This means it can scratch almost every other mineral. Only diamond (which is a 10 on the Mohs scale) is harder.
Because of its extreme hardness, a type of corundum called emery is often used as an abrasive. You might find it on sandpaper or on big tools that shape metals, plastics, and wood. Emery isn't pretty enough to be a gemstone; it's a black, grainy form of corundum mixed with other minerals like magnetite, hematite, or hercynite.
Besides being super hard, corundum is also quite heavy for a clear mineral made of lighter elements like aluminum and oxygen. It has a density of about 4.02 g/cm3 (251 lb/cu ft).
Where Does Corundum Come From?

Corundum can be found in different types of rocks, especially those that have been changed by heat and pressure (called metamorphic rocks), like mica schist, gneiss, and some marbles. It also appears in certain igneous rocks that have low amounts of silica, such as syenite and nepheline syenite.
Sometimes, you'll find corundum in large crystals within pegmatites (which are very coarse-grained igneous rocks) or near ultramafic rocks. Because it's so hard and doesn't break down easily, corundum crystals are often found washed into stream and beach sands.
The biggest natural corundum crystal ever found was huge! It measured about 65 cm × 40 cm × 40 cm (26 in × 16 in × 16 in) and weighed 152 kg (335 lb). That's like a small person! However, even bigger ones have been made in labs.
Famous Corundum Discoveries
Corundum used for abrasives (like emery) is mined in places like Zimbabwe, Pakistan, Afghanistan, Russia, Sri Lanka, and India. In the past, it was mined in North Carolina, USA, and Craigmont, Ontario, Canada. Emery-grade corundum is also found on the Greek island of Naxos and near Peekskill, New York, USA. Today, much of the abrasive corundum is made artificially from bauxite.
Interestingly, ancient corundum axes dating back to 2500 BC have been found in China, from the Liangzhu culture and Sanxingcun culture. This shows that people have known about and used corundum for a very long time!
Synthetic Corundum
Scientists and engineers have learned how to grow corundum crystals in laboratories. This is called "synthetic corundum."
Early Attempts to Create Corundum
- In 1837, a person named Marc Antoine Gaudin made the first artificial rubies. He did this by heating alumina (aluminum oxide) to a very high temperature and adding a tiny bit of chromium to give it color.
- In 1847, J. J. Ebelmen made clear artificial sapphires by reacting alumina in boric acid.
- Later, in 1877, Frenic and Freil made crystal corundum that could be cut into small stones. Frimy and Auguste Verneuil also made artificial ruby by melting BaF
2 and Al
2O
3 with a little chromium at temperatures over 2,000 °C (3,630 °F).
The Verneuil Process: Making Gems in a Lab
In 1903, Verneuil announced a way to make synthetic rubies on a large scale using a method called the flame fusion process. This method is still used today!
The Verneuil process allows factories to create perfect, single-crystal sapphire and ruby gems that are much bigger than what you usually find in nature. Other ways to grow high-quality synthetic corundum include flux-growth and hydrothermal synthesis. Because these methods are relatively simple, a lot of synthetic corundum crystals are available today, costing much less than natural stones.
Benefits of Man-Made Corundum
Making corundum in a lab can be better for the environment than mining it. It helps avoid destructive mining practices and saves natural resources. However, making synthetic corundum uses a lot of energy, which can contribute to carbon emissions if fossil fuels are used. Also, some chemicals are involved in the process that need to be handled carefully.
What is Corundum Used For?
Besides being used as beautiful gemstones, synthetic corundum has many important uses:
- Mechanical parts: It's used to make strong tubes, rods, bearings, and other machine parts.
- Scratch-resistant optics: Because it's so hard, it's used for scratch-resistant watch crystals and windows for instruments on satellites and spacecraft. It's great for space because it's clear in both ultraviolet and infrared light.
- Lasers: It's a key component in some lasers. For example, the main mirrors for the KAGRA gravitational wave detector are 50 lb (23 kg) sapphires, and Advanced LIGO considered using 40 kg (88 lb) sapphire mirrors.
- Armor: Corundum is also used to develop ceramic armor because of its incredible hardness.
The Science Behind Corundum's Strength
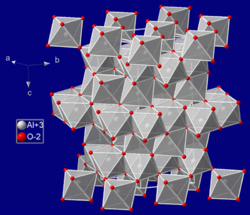
Corundum crystals have a special internal arrangement of atoms called a Trigonal symmetry. Imagine tiny building blocks (called unit cells) that repeat perfectly. In corundum, these blocks are arranged in a way that gives it its amazing strength.
How Corundum's Structure Makes it Strong
Inside corundum, the oxygen atoms are packed very closely together in a slightly twisted hexagonal close packing arrangement. The aluminum atoms then fit into specific spaces between these oxygen atoms. This precise arrangement, where two out of every three possible spaces are filled by aluminum, makes the crystal incredibly tough and hard.
The toughness of corundum can change depending on how smooth its surface is and the direction you're looking at its crystal structure. Synthetic crystals can be tougher than natural ones.
Scientists measure the strength of materials in different ways. For corundum (sapphire), its "Young's modulus" (which tells you how stiff a material is) is often around 345 GPa. This value can change a bit with temperature. Corundum also has a high "shear modulus" (145 GPa) and "bulk modulus" (240 GPa), which means it's very resistant to twisting and being compressed.
Even when corundum is made from many tiny crystals pressed together (called polycrystalline corundum), it can still be very strong. If these tiny crystals are very small (around 0.55-0.7 micrometers) and treated with a special process, they can have a bending strength between 600 and 900 MPa.
Structure Type
Because corundum is so common and its atomic structure is so stable, its structure has become a model for how other two-part and three-part chemical compounds are arranged. This is known as the corundum type structure.
See also
 In Spanish: Corindón para niños
In Spanish: Corindón para niños
 | Laphonza Butler |
 | Daisy Bates |
 | Elizabeth Piper Ensley |