Cottonmouth facts for kids
Quick facts for kids Cottonmouth |
|
|---|---|
 |
|
| An adult Florida cottonmouth (A. p. conanti) at Cincinnati Zoo | |
| Conservation status | |
| Scientific classification |
|
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Viperidae |
| Genus: | Agkistrodon |
| Species: |
A. piscivorus
|
| Binomial name | |
| Agkistrodon piscivorus (Lacépède, 1789)
|
|
 |
|
| Script error: The function "autoWithCaption" does not exist. | |
Script error: No such module "Check for conflicting parameters".
The cottonmouth (scientific name: Agkistrodon piscivorus) is a type of pit viper snake found in the southeastern United States. It's the only snake in the world that lives both on land and in water (semiaquatic). It's also the only venomous water snake in North America. Adult cottonmouths are large and can deliver a painful bite. When they feel threatened, they often coil up and show their fangs. They live near water, especially in slow-moving lakes, streams, and marshes. They are strong swimmers and can even swim in the ocean, but they are not true sea snakes. They have successfully made homes on islands along the Atlantic and Gulf coasts.
The name Agkistrodon comes from Greek words meaning "fish-hook" and "tooth." The word piscivorus comes from Latin words meaning "fish" and "eat greedily." So, its scientific name means "hook-toothed fish-eater." People also call this snake by other names like water moccasin, swamp moccasin, or black moccasin. Many of these names come from how the snake acts when it's scared: it opens its mouth wide to show the bright white inside. This white mouth looks like cotton, which is why it's called a cottonmouth. There are three types (subspecies) of cottonmouths.
Contents
- Cottonmouth Snake: Appearance and Features
- Common Names for the Cottonmouth
- Where Cottonmouths Live
- Cottonmouth Conservation Status
- Cottonmouth Habitat
- Cottonmouth Behavior
- Cottonmouth Feeding Habits
- Cottonmouth Predators
- Cottonmouth Reproduction and Life Cycle
- Cottonmouth Venom and Safety
- Cottonmouth Subspecies
- Images for kids
- See also
Cottonmouth Snake: Appearance and Features
The cottonmouth is the biggest snake in its group, Agkistrodon. Adult snakes are usually over 80 centimeters (about 31 inches) long. Females are typically smaller than males. The average weight for males is about 290 to 580 grams (10 to 20 ounces), and for females, it's about 200 to 250 grams (7 to 9 ounces). Some cottonmouths, especially in the eastern areas, can grow to be over 180 centimeters (about 6 feet) long. The largest cottonmouth ever recorded was 188 centimeters (about 6.2 feet) long.
Their heads are wide and clearly separate from their necks. The snout (nose area) is blunt. Most cottonmouths are dark brown or black. However, some can be brown, gray, tan, or yellowish-olive. They have 10 to 17 dark crossbands (stripes) on their bodies. These stripes often have black edges and are lighter in the middle. As the snakes get older, these patterns fade, and they become a more solid color like olive-brown, grayish-brown, or black.
The belly of the snake is usually white, yellowish-white, or tan with dark spots. The head is typically a plain brown color. Cottonmouths in the eastern areas have a wide, dark stripe behind their eyes. The underside of their head is usually whitish or cream.
Young cottonmouths have brighter patterns with dark stripes on a lighter background, which can be tan, brown, or reddish-brown. The tip of their tail is usually yellow, then becomes greenish-yellow, and finally black in adults. Young snakes wiggle their yellow tail tips to attract prey, like a fishing lure.
People often confuse cottonmouths with copperhead snakes. Young cottonmouths and copperheads look very similar. However, cottonmouths have wide, dark stripes on the sides of their heads that go back from their eyes. Copperheads only have a thin, dark line. Water snakes from the genus Nerodia also look similar. They are thick-bodied with large heads. But water snakes have round pupils (the black part of the eye), no heat-sensing pit near their nose, and different scale patterns.
Common Names for the Cottonmouth
Here are some common names for the Agkistrodon piscivorus snake:
- aquatic moccasin
- black moccasin
- black snake
- black water viper
- blunt-tail moccasin
- Congo
- copperhead
- cottonmouth
- cotton-mouthed snake
- cottonmouth rattler
- cottonmouth water moccasin
- gaper
- gapper
- highland moccasin
- lake moccasin
- lowland moccasin
- mangrove rattler
- moccasin
- moccasin snake
- North American cottonmouth snake
- North American water moccasin
- North American water viper
- pond moccasin
- pond rattler
- river moccasin
- river rattler
- rusty moccasin
- saltwater rattler
- short-tailed moccasin
- short-tail rattler
- small-tailed cottonmouth
- snap-jaw
- stub-tail
- stub-tail snake
- stump moccasin
- stump-tail moccasin
- stump-tail viper
- swamp lion
- swamp moccasin
- swamp rattler
- Texas moccasin
- trap jaw
- Troost's moccasin
- true horn snake
- true water moccasin
- viper
- water copperhead
- water mamba
- water moccasin
- water mokeson
- water pilot
- water pit rattler
- water pit viper
- water rattlesnake
- water viper
- white-mouth moccasin
- white-mouth rattler
- worm-tailed viper
Where Cottonmouths Live
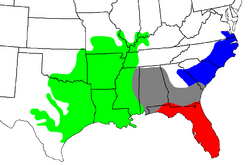
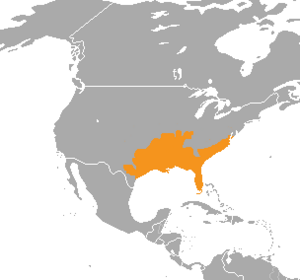
Cottonmouths are found in the eastern United States. Their range stretches from the Great Dismal Swamp in southeastern Virginia, south through Florida, and west to Arkansas, eastern and southern Oklahoma, and western and southern Georgia. They are also found in Alabama, Illinois, Indiana, Kentucky, Louisiana, Mississippi, Missouri, North Carolina, South Carolina, and Tennessee.
In Georgia, they live in the southern half of the state. Their range also includes the Ohio River Valley as far north as southern Indiana. They live on many barrier islands off the coasts of these states.
Cottonmouth Conservation Status
The cottonmouth is listed as a species of "least concern" by the IUCN Red List. This means they are not currently in danger of disappearing. They are widespread and have a large population. Even though people have hunted them and drained their wetland homes, they are still common in many areas.
However, in Indiana, the cottonmouth is listed as an endangered species. This means they are at high risk of becoming extinct in that state.
Cottonmouth Habitat
The cottonmouth is the most aquatic (water-loving) snake in its group. It usually lives near water bodies like creeks, streams, marshes, swamps, and the edges of ponds and lakes. They prefer shallow, slow-moving water. They are usually not found in fast, deep, or cold water. Their homes include lowland swamps, lakes, rivers, and even irrigation ditches and canals.
They can also live in brackish water (a mix of fresh and salt water) and are sometimes seen swimming in salt water. They have been very successful at living on coastal barrier islands. Even on these islands, they prefer freshwater marshes.
Cottonmouths are not limited to just water habitats. They can also be found in drier places like palmetto thickets, pine forests, and even prairies. Some large snakes have been found more than a mile (1.6 km) away from water.
Cottonmouth Behavior

When cottonmouths encounter people, about half of them try to get away. Most of them (about 78%) will show off their threat display to scare people away. They are most likely to bite if they are picked up or handled.
When a cottonmouth feels very stressed or threatened, it does a special threat display. It vibrates its tail and throws its head back, opening its mouth wide to show the bright white inside. It often makes a loud hiss. Its neck and the front part of its body are pulled into an S-shape, ready to strike. This behavior is why it's called "cottonmouth" or "gaper." If something touches its mouth, it might snap its jaws shut, which is why it's also called "trap jaw" in some places.
Other ways they defend themselves include flattening their body. They can also release a strong, smelly liquid from glands near their tail. This musk smells like a billy goat.
Harmless water snakes (genus Nerodia) are often mistaken for cottonmouths. Water snakes are also thick-bodied with large heads and can be aggressive. But they act differently. Water snakes usually quickly swim away into the water. Cottonmouths, however, often stand their ground with their threat display. Also, water snakes do not vibrate their tails when they are excited. Cottonmouths usually hold their heads at about a 45-degree angle when they swim or crawl.
Cottonmouths have very strong, muscular bodies. This makes them hard to hold. They can be active during the day and at night. On sunny days, they usually rest in the shade. In the morning or on cool days, they like to bask in the sun to warm up. They often come out at sunset to warm themselves on warm ground, like sidewalks or roads. Then, they become very active throughout the night, swimming or crawling. It's a common myth that they can't bite underwater, but they actually can.
In northern areas, cottonmouths hibernate (sleep through the winter). They are one of the last snakes to find shelter, often staying active until the first heavy frosts. Then, they move to higher ground and can be found in rotting pine stumps. In southern areas, their hibernation might be very short or not happen at all.
Cottonmouth Feeding Habits
Cottonmouths are carnivores, meaning they eat meat. Their diet includes small mammals, birds, amphibians, fish, other snakes, small turtles, and even baby alligators. They have also been known to eat other cottonmouths. Most of their diet is made up of fish and frogs. Young snakes sometimes eat insects. They often eat catfish, but the sharp spines can sometimes hurt them. They seem to avoid eating toads of the genus Bufo.
Cottonmouths are opportunistic eaters, meaning they eat whatever they can find easily. They sometimes eat carrion (dead animals), which is unusual for snakes. They have been seen eating fish heads and guts thrown from docks. They also take advantage when water bodies dry up in summer or fall. This concentrates fish and tadpoles, making them easy prey.
When hunting fish, they corner them in shallow water, often against the bank or under logs. They are surprisingly not very good at catching live or dead fish underwater.
Young cottonmouths have yellowish or greenish tail tips. They use these to "lure" prey. They wiggle the tail tip to attract animals like frogs and lizards close enough to strike. This luring behavior usually happens during the daytime. In Florida's coastal islands, cottonmouths often scavenge for dead fish that birds have dropped. Their diet there is mostly fish.
Cottonmouth Predators
Cottonmouths are hunted by several animals. These include snapping turtles, falcons, American alligators, horned owls, eagles, red-shouldered hawks, loggerhead shrikes, and large wading birds like herons, cranes, and egrets.
Other snakes that eat snakes (called ophiophagous snakes), including other cottonmouths, also prey on them. However, some kingsnakes, which are known for eating other snakes, might be hesitant to attack cottonmouths. Cottonmouths have a special defense against snake-eating predators: they raise a loop of their body off the ground and can throw it towards the attacker. This is called body-bridging.
Cottonmouth Reproduction and Life Cycle
Cottonmouths give birth to live young, meaning they don't lay eggs. Females usually have litters of 1 to 16 babies, but sometimes as many as 20. Litters of six to eight babies are most common. Newborn cottonmouths are 22–35 centimeters (about 8.5–14 inches) long. If there's good weather and plenty of food, they grow quickly. Females can start having babies when they are less than three years old and about 60 centimeters (2 feet) long. The young are usually born in August or September. Mating can happen during any of the warmer months.
Male cottonmouths sometimes fight each other to win the right to mate with females. This is a common behavior in many viper species.
There have been a few reports of female cottonmouths protecting their newborn babies. They might stay near their young and stand their ground if an intruder comes close.
How Cottonmouths Can Reproduce Alone
Cottonmouths can reproduce through a special process called facultative parthenogenesis. This means they can switch from having babies with a male (sexual reproduction) to having babies on their own without a male (asexual reproduction). This is rare in snakes but has been observed in both cottonmouths born in captivity and those found in the wild.
Cottonmouth Venom and Safety
The venom of the cottonmouth is strong and can destroy body tissue. While deaths from cottonmouth bites are rare, a bite can cause lasting scars and sometimes require serious medical procedures. Unlike some rattlesnake venoms, cottonmouth venom does not usually affect the nervous system or cause reactions throughout the body. Bites can be treated effectively with an antivenom called CroFab. This antivenom is made using venom from several types of American pit vipers, including the cottonmouth.
Bites from cottonmouths happen fairly often in the lower Mississippi River Valley and along the Gulf of Mexico coast. However, fatalities are very uncommon. For example, in Florida, there were three recorded deaths from cottonmouth bites in 1934, but no more after that year. Many people who spend a lot of time in cottonmouth habitats report never being bitten, even when they almost step on the snakes. This suggests that cottonmouths are not usually aggressive unless provoked.
The average amount of venom a cottonmouth can deliver is about 125 milligrams (dried). The exact amount that would be deadly to a human is not known, but it's estimated to be around 100–150 milligrams.
Common symptoms of a cottonmouth bite include bruising and swelling. The pain is usually worse than a copperhead bite but less severe than a rattlesnake bite. Blisters are less common than with rattlesnake bites, but tissue damage can occur. The venom has strong properties that can cause severe tissue destruction.
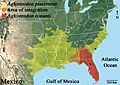
Cottonmouth Subspecies
| Subspecies | Taxon author | Common name | Geographic range |
|---|---|---|---|
| A. p. conanti | Gloyd, 1969 | Florida cottonmouth | The United States, in extreme southern Georgia and virtually all of the state of Florida, including many of the islands off the coast |
| A. p. leucostoma | (Troost, 1836) | Western cottonmouth | The United States, from southern Alabama along coast of the Gulf of Mexico, including many offshore islands, to southeastern and central Texas, and north to Oklahoma, Missouri, Illinois, and Indiana |
| A. p. piscivorus | (Lacépède, 1789) | Eastern cottonmouth | The United States in Delmarva Peninsula, the Atlantic Coastal Plain and lower Piedmont of North and South Carolina, including the banks, peninsulas, and islands along the Atlantic coast, and west across Georgia |
Images for kids
-
Geographic distribution of the two species of cottonmouth, Agkistrodon piscivorus and Agkistrodon conanti
-
A cottonmouth in water in Tennessee – the high position in the water and upward-tilted head can help distinguish it from Nerodia watersnakes such as the common watersnake, although there is substantial similarity.
-
A common watersnake (Nerodia sipedon) swimming – a nonvenomous species often mistaken for the cottonmouth
See also
 In Spanish: Boca de algodón para niños
In Spanish: Boca de algodón para niños