History of the Haber process facts for kids
The history of the Haber process tells the story of a super important invention from the early 1900s. This process helps us create ammonia from the air around us. Ammonia is then used to make many things, like fertilizers for growing food and explosives. It's one of the most important industrial inventions of the last century!
Long before factories existed, farmers used natural ways to make their crops grow better. They knew that adding things like animal waste to the soil helped plants get important nutrients. In the 1840s, a scientist named Justus von Liebig discovered that nitrogen was one of these key nutrients. Scientists also knew that nitrogen made up most of the air, but they didn't know how to turn it into useful forms easily.
Then, in 1909, a German chemist named Fritz Haber found a way to "fix" nitrogen from the air in his lab. This was a huge step! It had amazing uses for the military, factories, and farming. Just five years later, in 1913, a team from a company called BASF, led by Carl Bosch, turned Haber's lab idea into a big industrial process. Sometimes, it's called the Haber–Bosch process.
This new way to make nitrogen products helped Germany during World War I. Even when they couldn't get natural sources like guano, they could still make gunpowder and explosives. After the war, making ammonia became much cheaper. This helped modern farming grow and supported the world's growing population. During World War II, the Haber process also helped Nazi Germany make fuel, reducing their need to import oil.
Today, the Haber process and similar methods make almost all the synthetic ammonia used globally – over 100 million tons each year! Nitrogen fertilizers like urea and ammonium nitrate are vital for modern farming. They help feed at least two billion people around the world. However, these factories also have a big impact on the environment. About half of the nitrogen from these fertilizers isn't used by plants. It can end up in rivers or in the air.
Finding Nitrogen Before Haber
For hundreds of years, farmers understood that plants needed certain nutrients to grow. Different cultures found different ways to fertilize their land. In China, for example, human waste was used in rice fields. Justus von Liebig, a German chemist, even said that England "stole" millions of animal skeletons from Europe to get phosphorus for fertilizer! In Paris, people collected huge amounts of horse manure every year for city gardens. In the 1800s, bison bones from the American West were sent to factories on the East Coast to make phosphorus fertilizers.
From the 1820s to the 1860s, the Chincha Islands off Peru were famous for their high-quality guano (bird droppings). Peru exported this guano to the United States, France, and the United Kingdom. This "guano-boom" made Peru's economy grow a lot for a few decades. But eventually, all 12.5 million tons of guano were used up.
Scientists then started looking for other fertilizer sources. The Atacama Desert, which was part of Peru at the time, had large amounts of saltpeter (sodium nitrate). At first, saltpeter wasn't used much for farming. But then, chemists found a way to clean it to make gunpowder. Saltpeter could also be turned into nitric acid, which was used to make powerful explosives like nitroglycerine and dynamite. As more saltpeter was exported, tensions grew between Peru and its neighbors.
In 1879, Bolivia, Chile, and Peru fought a war over the Atacama Desert. This was called the "Saltpeter War." Chile quickly defeated Bolivia and Peru. By 1881, Chile controlled the saltpeter mines in the Atacama Desert. The use of Chilean saltpeter for farming quickly increased, and people in Chile became much wealthier.
However, new technologies in Europe soon changed things. By the 1900s, the minerals from this region provided very little of the world's nitrogen.
A Growing Need for Nitrogen
In the late 1800s, chemists like William Crookes predicted that the world would soon run out of enough nitrogen compounds. This was a problem because nitrogen was needed for both fertilizers and explosives.
Scientists already knew that ammonia was a nitrogen compound. Early attempts to create ammonia were made in 1795 by Georg Friedrich Hildebrandt. Many others tried throughout the 1800s.
In the 1870s, ammonia was just a leftover product from making gas for lighting. Later, its importance became clear. By the 1900s, factories started changing their equipment to make ammonia from coke. Still, they couldn't make enough to meet the demand.
In 1900, Chile, with its saltpeter deposits, produced two-thirds of all the fertilizer on Earth. But these deposits were quickly running out. Also, a few companies controlled the industry, so the price of saltpeter kept going up. To make sure there was enough food for Europe's growing population, a new, cheaper, and reliable way to get ammonia was desperately needed.
Germany faced a big challenge with food supply. Its soil wasn't very good, and the country didn't have a large empire to get resources from. Germany used a lot of Chilean saltpeter, importing 350,000 tons in 1900. Twelve years later, this jumped to 900,000 tons. The United States was in a better position because of the Guano Islands Act, which allowed them to claim islands with guano.
Between 1890 and 1900, chemistry made many advances. More scientists tried to fix nitrogen from the air. In 1895, German chemists Adolf Frank and Nikodem Caro found a way to react calcium carbide with nitrogen to make calcium cyanamide, which could be used as a fertilizer. Factories started using this Frank-Caro process in 1905. By 1918, 35 factories were making 325,000 tons of nitrogen each year. However, this process used a lot of electricity and needed more workers than the Haber process. Today, cyanamide is mostly used to kill weeds.
Wilhelm Ostwald, a top German chemist, tried to make ammonia in 1900 using his own invention. He got BASF interested, and they asked Carl Bosch, a new chemist, to check his device.
In 1901, Henry Le Chatelier successfully made ammonia from air. He even got a patent and believed he could get better results by increasing the pressure. But when one of his assistants died in an accidental explosion, Le Chatelier stopped his research.
In 1905, a Norwegian physicist named Kristian Birkeland, with money from industrialist Samuel Eyde, developed the Birkeland–Eyde process. This process also fixed nitrogen from the air. However, it needed a huge amount of electricity, so it could only be built in places with cheap power, like Norway. The company Norsk Hydro was started in 1905 to use this new process. By 1913, Norsk Hydro's factories were making 12,000 tons of nitrogen.
Other similar processes were developed around this time, but they were all quickly replaced by the cheaper Haber process.
A New Way to Make Ammonia
In 1905, German chemist Fritz Haber published a book about how chemistry could be used in factories. In his book, Haber included the results of his study on how ammonia forms:
- N2 (gas) + 3 H2 (gas)
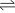 2 NH3 (gas) - heat
2 NH3 (gas) - heat
He found that at very high temperatures (1000°C) and with an iron catalyst, small amounts of ammonia were made from nitrogen and hydrogen gases. These results didn't make him want to continue. But in 1907, a scientific competition between Haber and Walther Nernst pushed Haber to focus on nitrogen fixation. A few years later, Haber used Nernst's findings and his own knowledge of high pressure chemistry to create a new way to fix nitrogen. He figured out that a good ammonia production system needed to:
- Work at very high pressure (around 200 times normal air pressure).
- Use one or more catalysts to speed up the ammonia creation.
- Operate at a high temperature (between 500°C and 600°C) for the best results with the catalyst.
- Since only about 5% of the nitrogen and hydrogen reacted each time they went through the machine:
- Separate the ammonia from the other gases by turning it into a liquid.
- Remove the liquid ammonia continuously.
- Send the unreacted nitrogen and hydrogen back into the machine.
- Reuse the heat that was produced.
To handle the high pressure, Haber got help from Robert Le Rossignol, who designed the special equipment. In early 1909, Haber found that osmium could work as a catalyst. Later, he found that uranium and even iron, nickel, manganese, and calcium could also work. The reaction that makes ammonia also produces heat. Haber's team developed a system to reuse this heat to warm up the gases before they entered the machine.
In March 1909, Haber showed his lab colleagues that he had finally found a process that could fix enough nitrogen from the air to be used in factories.
Even though BASF got a patent for the Haber process, August Bernthsen, a research director at BASF, wasn't sure it was useful. He didn't think BASF should get involved. Bernthsen believed no factory equipment could handle such high pressure and temperature for long enough to make it worth the money. Also, he thought the catalyst might stop working, and osmium was very rare.
However, Carl Engler, a chemist and professor, wrote to BASF President Heinrich von Brunck to convince him to talk to Haber. Von Brunck, along with Bernthsen and Carl Bosch, went to Haber's lab to see if BASF should try to make the process industrial. When Bernthsen heard they needed equipment that could handle at least 100 atm (about 10 times normal air pressure), he exclaimed, "One hundred atmospheres! Just yesterday a pressure cooker exploded on us at seven atmospheres!" Before deciding, von Brunck asked for Bosch's opinion.
Carl Bosch had worked with metallurgy (working with metals) before. His father had a workshop at home where young Carl learned to use different tools. He had been working on nitrogen fixation for years but hadn't gotten good results. He knew that processes using electric furnaces, like the Birkeland–Eyde process, needed huge amounts of electricity, making them too expensive outside of Norway. To keep growing, BASF needed a cheaper way to fix nitrogen. Bosch said, "I think it can work. I know exactly what the steel industry can do. We should risk it."
In July 1909, BASF employees came to check Haber's success again. The lab equipment was fixing nitrogen from the air, turning it into liquid ammonia, at a rate of about 250 milliliters every two hours. BASF decided to make the process industrial, even though they were already working with Norsk Hydro on another process. Carl Bosch, who would lead the industrialization of the process, said that the main reason BASF went ahead was the improved efficiency of the catalyst.


