Silicate minerals facts for kids
Silicate minerals are a huge group of minerals that make up most of the Earth's crust—about 90 percent! They are called "silicates" because they are built from special groups of silicon and oxygen atoms. These minerals are found everywhere, from the rocks in mountains to the sand on beaches.
One very common silicate mineral is quartz. You might know it as the main part of sand. Quartz is a form of silicon dioxide (SiO2), which is also considered a silicate.
Silicate minerals form in many ways over billions of years. This includes processes like rocks melting and then cooling, changing under heat and pressure (metamorphism), and breaking down from weather.
Even living things help create silicates! For example, tiny ocean creatures called diatoms build their hard outer shells from silica they take from seawater. When these diatoms die, their shells sink and become a big part of deep ocean sediment. This sediment can eventually form a soft rock called diatomaceous earth.
Contents
- What are Silicate Minerals Made Of?
- How Do We Group Silicate Minerals?
- Nesosilicates: The "Island" Silicates
- Sorosilicates: The "Heap" Silicates
- Cyclosilicates: The "Ring" Silicates
- Inosilicates: The "Chain" Silicates
- Phyllosilicates: The "Sheet" Silicates
- Tectosilicates: The "Framework" Silicates
- Images for kids
- See also
What are Silicate Minerals Made Of?
Most silicate minerals are ionic compounds. This means they are made of atoms with electrical charges. The main parts of these minerals are groups of silicon and oxygen atoms.
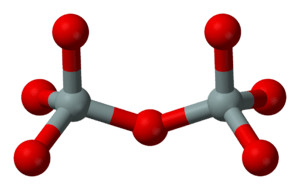
Imagine a silicon atom in the middle, surrounded by four oxygen atoms. This shape is called a tetrahedron, like a small pyramid. These silicon-oxygen pyramids are the basic building blocks of all silicate minerals. Sometimes, two pyramids can share an oxygen atom, connecting them together. If an oxygen atom isn't shared, it has a negative charge, which helps balance the mineral's overall charge.
Sometimes, other atoms, like aluminum, can take the place of a silicon atom in these pyramids. When this happens, it can change the mineral's charge. For example, in a mineral called orthoclase, aluminum atoms replace some silicon atoms. This gives the mineral a negative charge, which is then balanced by positive potassium atoms.
How Do We Group Silicate Minerals?
Scientists group silicate minerals based on how their silicon-oxygen pyramids are connected. There are seven main groups:
| Main Group | How Pyramids Connect | Example |
|---|---|---|
| Nesosilicates | Separate pyramids | olivine |
| Sorosilicates | Double pyramids | epidote |
| Cyclosilicates | Rings of pyramids | tourmaline |
| Inosilicates | Single chains of pyramids | pyroxene group |
| Inosilicates | Double chains of pyramids | amphibole group |
| Phyllosilicates | Flat sheets of pyramids | micas, clays |
| Tectosilicates | 3D frameworks of pyramids | quartz, feldspars |
For tectosilicates, if some silicon atoms are replaced by other atoms like aluminum, the mineral might need extra positive atoms to balance its charge.
Nesosilicates: The "Island" Silicates
Nesosilicates (from the Greek word for "island") have silicon-oxygen pyramids that are completely separate from each other. They are like tiny islands connected only by other atoms that hold them together.
Some examples of nesosilicates include:
- Olivine group: Like Forsterite (Mg2SiO4) and Fayalite (Fe2SiO4).
- Garnet group: Such as Pyrope (Mg3Al2(SiO4)3) and Almandine (Fe3Al2(SiO4)3).
- Zircon group: Including Zircon (ZrSiO4).
- Al2SiO5 group: Like Andalusite, Kyanite, and Sillimanite.
- Topaz (Al2SiO4(F,OH)2).
Sorosilicates: The "Heap" Silicates
Sorosilicates (from the Greek word for "heap" or "mound") have silicon-oxygen pyramids that are joined in pairs. They share one oxygen atom, forming a double pyramid structure.
Examples of sorosilicates are:
- Hemimorphite (Zn4(Si2O7)(OH)2·H2O).
- Lawsonite (CaAl2(Si2O7)(OH)2·H2O).
- Epidote group: Such as Epidote (Ca2(Al,Fe)3O(SiO4)(Si2O7)(OH)) and Zoisite (Ca2Al3O(SiO4)(Si2O7)(OH)).
Cyclosilicates: The "Ring" Silicates
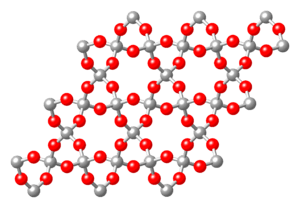
Cyclosilicates (from the Greek word for "circle") have silicon-oxygen pyramids linked together in rings. These rings can have different numbers of pyramids, like 3, 4, 6, or even 9.
Some examples of cyclosilicates include:
- Benitoite (BaTi(Si3O9)), which has 3-member rings.
- Beryl (Be3Al2(Si6O18)), a well-known gemstone, has 6-member rings.
- Tourmaline (a complex mineral with 6-member rings).
- Cordierite ((Mg, Fe)2Al4Si5O18).
Inosilicates: The "Chain" Silicates
Inosilicates (from the Greek word for "fibre") have silicon-oxygen pyramids linked together to form long chains. These can be single chains or double chains.
Single Chain Inosilicates
These minerals have a single line of silicon-oxygen pyramids linked together.
- Pyroxene group: A very common group of minerals. Examples include Enstatite (MgSiO3), Diopside (CaMgSi2O6), and Jadeite (NaAlSi2O6).
- Wollastonite (CaSiO3).
- Rhodonite (MnSiO3).
Double Chain Inosilicates
These minerals have two parallel chains of silicon-oxygen pyramids linked together.
- Amphibole group: Another very common group. Examples include Anthophyllite (Mg,Fe)7Si8O22(OH)2, Tremolite (Ca2Mg5Si8O22(OH)2), and Hornblende (a complex amphibole).
Phyllosilicates: The "Sheet" Silicates
Phyllosilicates (from the Greek word for "leaf") form flat, parallel sheets of silicon-oxygen pyramids. Because of this sheet-like structure, many of these minerals can be easily split into thin layers, like the pages of a book. All phyllosilicates contain water or hydroxyl (OH) groups.
Some examples of phyllosilicates are:
- Serpentine subgroup: Including Antigorite and Chrysotile (Mg3Si2O5(OH)4).
- Clay minerals: Such as Kaolinite (Al2Si2O5(OH)4) and Montmorillonite (a type of swelling clay).
- Talc (Mg3Si4O10(OH)2), which is very soft.
- Mica group: Like Biotite (K(Mg,Fe)3(AlSi3)O10(OH)2) and Muscovite (KAl2(AlSi3)O10(OH)2).
- Chlorite group: A common group found in metamorphic rocks.
Tectosilicates: The "Framework" Silicates
Tectosilicates, also called "framework silicates," have silicon-oxygen pyramids connected in a complex, three-dimensional network. This group makes up almost 75% of the Earth's crust!
Most tectosilicates also contain aluminum atoms mixed in with the silicon.
Important examples include:
- Quartz family: All forms of SiO2, like Quartz itself, Tridymite, and Cristobalite.
- Feldspar group: These are the most common minerals in the Earth's crust.
* Alkali feldspars: Such as Orthoclase (KAlSi3O8). * Plagioclase feldspars: A series of minerals ranging from Albite (NaAlSi3O8) to Anorthite (CaAl2Si2O8).
- Feldspathoid family: Minerals like Nepheline ((Na,K)AlSiO4) and Sodalite (Na8(AlSiO4)6Cl2).
- Zeolite family: These minerals have open structures with channels, which allows them to absorb water. Examples include Natrolite (Na2Al2Si3O10·2H2O) and Chabazite (CaAl2Si4O12·6H2O).
Images for kids
-
6 units [Si
6O
18], beryl (red: Si, blue: O)
See also
 In Spanish: Silicato (mineral) para niños
In Spanish: Silicato (mineral) para niños