Sulfuric acid facts for kids
Sulfuric acid, also called sulphuric acid, is a very strong acid. It is a chemical compound with the chemical formula H2SO4. Long ago, people called it "Oil of Vitriol."
Contents
What is Sulfuric Acid Like?
Sulfuric acid is a thick, clear liquid. It is very corrosive, meaning it can eat away at many materials. It can easily mix with water. It can also melt through some weaker substances.
A Look Back: History of Sulfuric Acid
The person who first discovered sulfuric acid was Jabir ibn Hayyan. He was the one who gave it the name "oil of vitriol."
In the past, sulfuric acid was made by mixing potassium nitrate with sulfur. This reaction created sulfur trioxide, which then dissolved in steam to make sulfuric acid. Later, this process was done inside lead containers.
The most common way to make sulfuric acid today is called the contact process. This method was invented in 1831 and is still used around the world.
How Sulfuric Acid is Made
Sulfuric acid is made using a method called the contact process. First, sulfur is burned to create sulfur dioxide. Then, the sulfur dioxide is changed into sulfur trioxide with the help of a catalyst. A catalyst is something that speeds up a chemical reaction without being used up itself.
After that, the sulfur trioxide reacts with sulfuric acid to make a substance called oleum. Finally, oleum is mixed with water to produce even more sulfuric acid.
Everyday Uses of Sulfuric Acid
Sulfuric acid is used in many different ways. In 2001, the world made 165 million tons of it! That's a lot of acid.
Here are some of its main uses:
- Making Fertilizers: It helps create important plant foods. For example, it reacts with phosphate rocks to make useful phosphates for fertilizers. It also helps make phosphoric acid, which is used in soda.
- Processing Ores: It helps separate valuable metals from rocks.
- Refining Oil: It plays a role in making gasoline and other oil products.
- Cleaning Wastewater: It helps treat dirty water to make it safe.
- Car Batteries: Lead acid batteries, like those in cars, use sulfuric acid as their electrolyte. This liquid helps the battery store and release electricity.
- Creating Other Chemicals: It's used to combine chemicals for many scientific and industrial purposes. For example, it reacts with ammonia to make ammonium sulfate, another important fertilizer.
Safety and Chemical Reactions
Sulfuric acid is very dangerous because it is so acidic and corrosive. It can cause serious burns if it touches your skin.
Here are some interesting facts about its reactions:
- With Sugar: If you mix concentrated sulfuric acid with sugar, it can turn the sugar into black carbon.
- With Water: When sulfuric acid is mixed with water, it becomes less harmful, but it is still corrosive. Always add acid slowly to water, not the other way around, because it creates a lot of heat!
- With Metals: It reacts with most metals to produce hydrogen gas and a metal sulfate. Some metals, like tin, can also produce sulfur dioxide.
- With Salt: It reacts with sodium chloride (table salt) to make hydrogen chloride gas.
Related pages
Images for kids
-
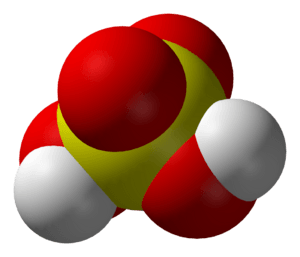
John Dalton's 1808 sulfuric acid molecule shows a central sulfur atom bonded to three oxygen atoms, or sulfur trioxide, the anhydride of sulfuric acid.
-
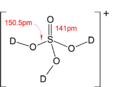
Solid state structure of the [D3SO4]+ ion present in [D3SO4]+[SbF6]−, synthesized by using DF in place of HF. (see text)
-
Rio Tinto with its highly acidic water
-
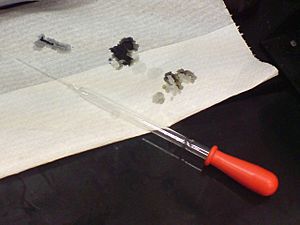
Acidic drain cleaners usually contain sulfuric acid at a high concentration which turns a piece of pH paper red and chars it instantly, demonstrating both the strong acidic nature and dehydrating property.
See also
 In Spanish: Ácido sulfúrico para niños
In Spanish: Ácido sulfúrico para niños