Liquid–liquid extraction facts for kids
Liquid–liquid extraction (LLE), also called solvent extraction, is a cool way to separate different substances. Imagine you have a mix of things, and you want to pull out just one part. This method uses two liquids that don't mix, like oil and water.
One liquid is usually water (which is polar), and the other is an organic solvent (which is non-polar). Substances move from one liquid to the other based on how well they dissolve in each. It's like some things prefer to hang out in the water, while others prefer the oil.
When a substance moves into the new liquid, that new liquid becomes the extract. The original liquid, which now has less of the substance, is called the raffinate. This technique is super common in science labs. Scientists use tools like separatory funnels to do it. It's often used after a chemical reaction to clean up the new chemicals.
The word partitioning often describes the science behind how substances divide between the two liquids. Solvent extraction can also mean separating something from a mix by dissolving it in a special solvent. This helps separate a soluble compound from something that doesn't dissolve.
In big industries, like those that work with metals, solvent extraction is used to separate and clean important metals. This includes things like uranium, plutonium, and rare earth elements. It's great because it can separate even very similar metals. After the metal is pulled into the organic liquid, it can be "stripped" back out into a clean water solution. Stripping is the opposite of extraction.
LLE is also used to make many everyday products. This includes fine organic compounds, perfumes, vegetable oils, and biodiesel. It's one of the first steps in separating many different things.
This method can even work with liquids that aren't water. For example, molten metals can be separated using molten salts. It's a versatile way to get pure substances from complex mixtures.
Contents
- How well does it work?
- Extraction methods
- How fast does it happen?
- Aqueous complexing agents
- Industrial process design
- Equipment
- Extracting metals
- See also
How well does it work?
Distribution ratio
When we do solvent extraction, we want to know how much of a substance moves into the new liquid. We use something called the distribution ratio (Kd) to measure this. It's simply the amount of the substance in the organic liquid divided by its amount in the water liquid.
This ratio can change based on things like temperature or how much of other chemicals are in the mix. When you shake two liquids that don't mix, substances that are more polar (like water) will dissolve better in the polar liquid. Substances that are less polar will dissolve better in the less polar liquid.
Sometimes, the distribution ratio is called the partition coefficient. While they are often used to mean the same thing, they can be different. This is because a substance might exist in more than one form in a liquid. The partition coefficient is fixed, but the distribution ratio can change with different conditions.
Separation factors
The separation factor helps us understand how well we can separate two different substances. Imagine you have two substances, like nickel and silver. If silver moves into the organic liquid 10 times better than nickel, then the separation factor for silver over nickel is 10. This number tells us how good the system is at pulling apart two specific things.
Decontamination factor
This factor shows how well a process removes something unwanted, like a contaminant, from a product. For example, if you start with a mix that has a lot of a contaminant, and your process removes most of it, the decontamination factor will be high. It tells you how much cleaner your product becomes.
Slopes of graphs
Scientists sometimes draw graphs to figure out how the extraction works. By looking at the "slope" of a line on a graph, they can learn about the chemical reactions happening during the extraction. For example, if the amount of a substance extracted doubles when you double a certain chemical, the slope on the graph would be two.
Measuring success
We measure the success of liquid–liquid extraction using separation factors and decontamination factors. A good way to understand how well an extraction column works is by looking at its liquid-liquid equilibrium (LLE) data. This data shows how a substance divides between the two liquids. It helps scientists make the process work even better.
Extraction methods
Single stage extractions
This is a common method used in small labs. It often involves a separating funnel. You mix the two liquids, let them settle, and then separate them.
Dispersive liquid–liquid microextraction (DLLME)
DLLME is a method to extract tiny amounts of organic compounds from water. You inject small amounts of special solvents into the water. Then, you spin the solution in a centrifuge to separate the layers. This is useful for finding things like pesticides in water.
Direct organic extraction
In this method, you mix a sample with an organic solvent like toluene or benzene. The organic parts of the sample dissolve in the solvent. Then, you use a separatory funnel to separate the layers. This can be used to extract proteins.
For example, you can extract anisole from water using ether. The anisole moves into the ether layer. Then, you can clean the ether layer by shaking it with sodium bicarbonate. This removes unwanted acids.
You can also extract caffeine from coffee beans or tea leaves this way. Soaking them in ethyl acetate pulls out the caffeine, leaving most of the flavor behind.
Multistage continuous processes
These methods are used in big factories, especially for separating metals like lanthanides. Because these metals are very similar, many steps are needed to separate them completely. In these processes, the liquids flow continuously in opposite directions through many stages. This helps to get a very pure product, even if each step only separates a little bit.
For a good process, the distribution ratio shouldn't be too high or too low. Often, there's a "scrubbing" section to remove unwanted metals and a "stripping" section to get the desired metal back.
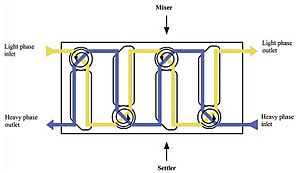
Mixer–settlers
Mixer-settlers are machines used in these multistage processes. Each unit has a part that mixes the liquids and then a part where they settle and separate by gravity. They are good for processes that need a longer time for the liquids to mix and separate. They take up a lot of space but don't need much height.
Centrifugal extractors
Centrifugal extractors are machines that mix and separate liquids in one unit. They spin liquids very fast, up to 6000 rotations per minute! This creates a huge surface area for the substances to move between the liquids. The spinning also helps separate the liquids quickly. These machines use less solvent and can extract substances very efficiently.
Extraction without chemical change
Some substances, like noble gases, can be extracted without any chemical reaction. This is the simplest type of solvent extraction. When two liquids that don't mix are shaken, the more polar substances go to the polar liquid, and the less polar ones go to the less polar liquid.
Sometimes, even without a clear reaction, the amount extracted can change with concentration. For example, carboxylic acids can form pairs (dimers) in the organic layer, which changes how much is extracted.
Solvation mechanism
This method uses a special organic solvent to pull out metals like uranium, plutonium, and rare earth elements from acid solutions. For example, the PUREX process, used in nuclear reprocessing, uses a solvent called tributyl phosphate (TBP) mixed with kerosene. Uranium forms a special complex with TBP and moves into the organic layer. To get the uranium back, it's mixed with a weak acid, which breaks the complex. Plutonium can be separated similarly.
Ion exchange mechanism
In this method, when one ion moves from the water liquid to the organic liquid, another ion moves in the opposite direction. This keeps the electrical charge balanced. Often, a hydrogen ion is the one that moves. Because of this, the amount extracted often depends on the pH (how acidic or basic the solution is).
For example, americium can be extracted using a combination of chemicals where hydrogen ions are exchanged. Another example is the extraction of zinc, cadmium, or lead using a special acid that helps them move into a non-polar solvent.
Ion pair extraction
You can also extract a metal by carefully choosing a "counterion." For instance, if there's a lot of nitrate in the water, americium can be extracted as a negatively charged nitrate complex if a special salt is added.
A common example for chemists is using a phase transfer catalyst. This is a charged substance that helps another ion move into the organic liquid. The ion then reacts and forms a new ion, which moves back to the water liquid. This process can make chemical reactions happen more easily.
Aqueous two-phase extractions
These systems use two water-based liquids that don't mix.
- Polymer–polymer systems: Both liquids are made from dissolved polymers. One is usually a polysaccharide (like dextran), and the other is Polyethylene glycol (PEG). These systems are good for cleaning proteins because they don't use harsh solvents. It can be tricky to separate these layers because they have similar densities.
- Polymer–salt systems: One liquid is a polymer (usually PEG), and the other is a strong salt solution. These are easier to separate than polymer-polymer systems. However, high salt concentrations can sometimes damage proteins.
- Ionic liquids systems: Recent research uses ionic liquids (salts that are liquid at low temperatures) for extraction without organic solvents.
DNA purification
DNA extraction is very important in modern science. Sometimes, samples have enzymes that can break down DNA. It's been shown that DNA pieces can move into the lighter liquid in a polymer-salt system. If chemicals that stop these enzymes are added, the enzymes move to the heavier liquid and are deactivated. This helps clean DNA while protecting it.
Food industry
Polymer-salt systems can separate small molecules like peptides and nucleic acids. These compounds often give food its flavor or smell. So, the food industry could use this to get specific flavors or remove unwanted ones. Caffeine used to be extracted from coffee and tea using liquid-liquid extraction, but now other methods are more common for large-scale production.
Analytical chemistry
In analytical chemistry, sometimes there are chemicals in a sample that would mess up the analysis. For example, if you're testing air quality, the coating on a filter might interfere. If the substance you want to test can be extracted into a non-polar solvent, and the interfering coating is polar, the coating will stay in the water layer. This helps get a clean sample for testing.
Purification of amines
Amines are chemicals that can act like bases. In acidic water, they get a positive charge and dissolve well. In basic water, they become neutral and prefer non-polar solvents. So, you can add a base to an amine solution, shake it with a non-polar solvent, and the amine will move into the non-polar layer. You can then separate the layers and get the pure amine. You can even reverse the process to move the amine back into a water layer.
Temperature swing solvent extraction
This is an experimental method used to remove salt from drinking water. It has been shown to remove a lot of salt and can even clean very salty water that other methods can't handle.
How fast does it happen?
It's important to know how fast a substance moves between the two liquids. Sometimes, changing the contact time can help you separate things better. For example, extracting palladium or nickel can be very slow compared to iron or silver.
Aqueous complexing agents
If there's another chemical in the water that can bind to the substance you want to extract, it can change how much of that substance moves into the organic liquid. For example, if you're extracting iodine, and there's iodide in the water, the iodine can react with the iodide to form a new ion. This changes the extraction process.
Industrial process design
In factories, the extraction process usually has a few steps:
- Extraction: The desired substances move from the water to the organic liquid.
- Scrubbing: Unwanted substances are removed from the organic liquid.
- Stripping: The desired substances are removed from the organic liquid, making it pure.
- Cleaning: The organic liquid is cleaned so it can be used again. For example, in nuclear plants, the used organic liquid is washed with sodium carbonate to remove impurities.
Equipment
While chemists in labs might use a separatory funnel, large factories use big machines. These include centrifugal contactors, Thin Layer Extraction, spray columns, pulsed columns, and mixer-settlers.
Extracting metals
Here are some examples of how liquid-liquid extraction is used to get different metals:
Cobalt
Cobalt can be extracted from hydrochloric acid using special organic chemicals like Alamine 336 or Cyanex 272.
Copper
Copper can be extracted using chemicals called hydroxyoximes. Some new ones are very good at separating copper from cobalt and nickel.
Neodymium
The rare earth element Neodymium is extracted using a chemical called di(2-ethyl-hexyl)phosphoric acid in hexane. This happens through an ion exchange process.
Nickel
Nickel can be extracted using di(2-ethyl-hexyl)phosphoric acid and tributyl phosphate in a hydrocarbon solvent.
Palladium and platinum
Special chemicals like dialkyl sulfides, tributyl phosphate, and alkyl amines are used to extract palladium and platinum.
Polonium
Polonium is made in nuclear reactors. After it's made, it's purified using a liquid-liquid extraction method with sodium hydroxide at very high temperatures.
Zinc and cadmium
Zinc and cadmium are extracted using an ion exchange process. A chemical called TPEN helps separate them. In a process called Zincex, zinc is separated from other metals using D2EHPA. To get the zinc back, sulfuric acid is used.
Lithium
Extracting Lithium is very important because of the high demand for lithium-ion batteries. Chemicals like TBP and FeCl
3 are often used to extract lithium from salty water (brine). Cyanex 272 can also be used. The way lithium is extracted is a bit different from other metals because it doesn't bond as strongly with the extracting chemicals.
See also
 In Spanish: Extracción líquido-líquido para niños
In Spanish: Extracción líquido-líquido para niños
- Fragrance extraction
- Dortmund Data Bank
- Non-random two-liquid model - (NRTL model) LL Phase Equilibrium Calculation
- UNIQUAC - LL Phase Equilibrium Calculation
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |