Inorganic chemistry facts for kids
Inorganic chemistry is a super interesting part of chemistry. It's all about studying everything that isn't "organic." Think of it as exploring the building blocks of our universe and how they combine!
Contents
What is Inorganic Chemistry?
In simple terms, inorganic chemistry looks at all the elements on the periodic table and the many different inorganic compounds they form. If you imagine chemistry as a huge playground, inorganic chemistry is the part where you learn about all the swings, slides, and monkey bars themselves, and how they connect, rather than just one specific game played on them.
Inorganic vs. Organic Chemistry
Organic chemistry mainly focuses on compounds that contain carbon, especially when carbon is bonded to hydrogen. Inorganic chemistry studies all the other elements and compounds. It even includes some carbon compounds, like carbon dioxide (CO2) or diamond, because they don't have carbon-hydrogen bonds.
What Do Inorganic Chemists Study?
Inorganic chemists explore a huge range of materials. They study metals, salts, minerals, and even water. They look at how these substances behave, how they react with each other, and what properties they have.
For example, they might study:
- How to make new materials for batteries or solar panels.
- How catalysts work to speed up chemical reactions.
- The role of metals in living things, like iron in your blood.
- The properties of superconductors, which can conduct electricity with no resistance.
Why is Inorganic Chemistry Important?
Inorganic chemistry is vital for many parts of our daily lives. It helps us understand:
- How fertilizers help plants grow.
- How medicines work in our bodies.
- How to purify drinking water.
- How to create new materials for computers, cars, and buildings.
It's a field that helps us understand the world around us, from the tiniest atoms to the largest rocks.
Related pages
Images for kids
-
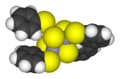
Tetrasulfur tetranitride, S4N4, is a main group compound that continues to intrigue chemists
-
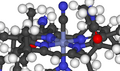
Organolithium reagents are most often found in polymeric form, such as n-butyllithium shown here
-
Decaborane is a powerfully toxic cluster compound of boron
-
Iron–sulfur clusters are central components of iron–sulfur proteins, essential for human metabolism
-
The octahedral cobalt centre of Vitamin B12
-
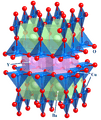
YBa2Cu3O7, or YBCO, is a high temperature superconductor able to levitate above a magnet when colder than its critical temperature of about 90 K (−183 °C)
-
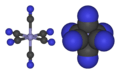
Crystal field theory explains why [FeIII(CN)6]3− has only one unpaired electron
-
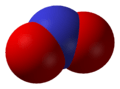
Nitrogen dioxide, NO2, exhibits C2v symmetry
See also
 In Spanish: Química inorgánica para niños
In Spanish: Química inorgánica para niños