Stable nuclide facts for kids
Imagine tiny building blocks that make up everything around us! These are called atoms. Inside every atom is a tiny center called the nucleus. The nucleus is made of even smaller particles: protons and neutrons, which together are called nucleons.
Sometimes, atoms of the same chemical element can have different numbers of neutrons. These different versions are called isotopes. A stable nuclide is a special kind of isotope whose nucleus is perfectly balanced and doesn't break apart. Unlike radionuclides (which are radioactive), stable nuclides don't give off radiation or change into other elements on their own. When we talk about a specific element, we often call these its stable isotopes.
There are 80 elements that have at least one stable isotope. In total, scientists have found 251 nuclides that seem to be stable and haven't been seen to decay with our current tools. Out of these 80 elements, 26 have only one stable isotope. These are known as monoisotopic elements. The other 56 elements have more than one stable isotope. Did you know that Tin has the most stable isotopes of any element? It has ten!
Contents
What Makes an Isotope Stable?
Most of the nuclides we find naturally are stable. There are about 251 stable ones. Plus, there are about 35 more that are radioactive but have very, very long half-lives. These long-lived ones are called primordial nuclides because they have been around since the Solar System formed about 4.5 billion years ago. If a nuclide's half-life is as long as or longer than Earth's age, a lot of it would still be here!
For example, 235U is a primordial radioisotope with a half-life of 700 million years. We can still find it today. Nuclides with shorter half-lives usually aren't found naturally unless they've been made recently. This can happen when they are products of other radioactive decays (like radium from uranium) or when cosmic rays hit Earth (like 14C made from nitrogen).
How Do Scientists Define Stability?
Some isotopes that we call "stable" (because we haven't seen them decay) are actually predicted to have extremely long half-lives. We're talking about 10 followed by 18 zeros years or even more! If scientists develop new, more sensitive equipment, they might eventually see these "stable" isotopes decay. If that happens, they would move from the "stable" list to the "radioactive" list.
For instance, 209Bi and 180W were once thought to be stable. But in 2003, scientists found that they actually undergo a very slow alpha decay. Even if they are found to be radioactive, they are still considered primordial if they've been around since the Earth formed.
Most stable isotopes on Earth were created in stars through a process called nucleosynthesis. This happened either during the Big Bang or in older stars before our Solar System was born. However, some stable isotopes on Earth also come from the decay of long-lived radioactive nuclides. These are called radiogenic isotopes.
How Many Stable Isotopes Does Each Element Have?
Out of all the known chemical elements, 80 of them have at least one stable nuclide. These include the first 82 elements, from hydrogen all the way to lead. The only two exceptions are technetium (element 43) and promethium (element 61), which don't have any stable nuclides at all. As of 2023, there are 251 known "stable" nuclides. "Stable" here means that their decay has never been observed. Their half-lives are simply too long to measure!
Here's a quick look at how many stable isotopes different elements have:
- One element (tin) has 10 stable isotopes.
- Five elements have 7 stable isotopes each.
- Seven elements have 6 stable isotopes each.
- Eleven elements have 5 stable isotopes each.
- Nine elements have 4 stable isotopes each.
- Five elements have 3 stable isotopes each.
- Sixteen elements have 2 stable isotopes each.
- Twenty-six elements have only 1 stable isotope. These are the monoisotopic elements.
On average, elements that have at least one stable isotope have about 3.1 stable isotopes.
Why Are Some Isotopes More Stable?
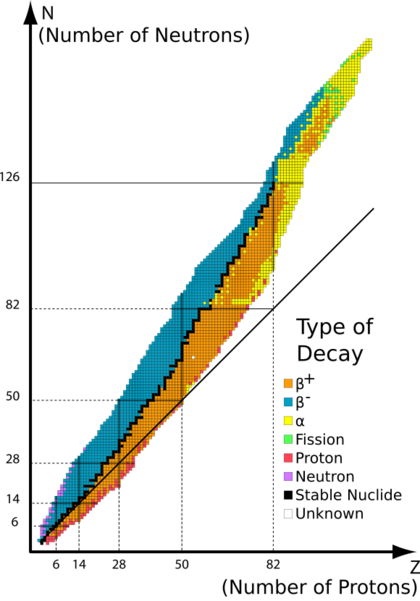
The stability of an isotope depends on a few things. One important factor is the balance between the number of protons and neutrons in its nucleus. Another factor is the presence of "magic numbers" of neutrons or protons. These magic numbers mean that the protons or neutrons fill up complete "shells" inside the nucleus, similar to how electrons fill shells around an atom. When these shells are full, the nucleus becomes extra stable. For example, tin has 50 protons, which is a magic number, and that's why it has so many stable isotopes.
Just like electrons prefer to be in pairs, protons and neutrons also prefer to be in pairs. This means that nuclei with an even number of protons and an even number of neutrons are usually more stable than those with odd numbers. This is why 150 of the 251 stable nuclides have both an even number of protons and an even number of neutrons. These "even-even" nuclides are so stable that they often need to undergo a rare process called double beta decay to change, which takes an incredibly long time.
On the other hand, only five of the 251 known stable nuclides have both an odd number of protons and an odd number of neutrons. These are hydrogen-2 (deuterium), lithium-6, boron-10, nitrogen-14, and tantalum-180m. Most odd-odd nuclei are unstable because they can easily decay into more stable even-even nuclei.
This also explains why elements with an odd number of protons tend to have fewer stable isotopes. Out of the 26 elements with only one stable isotope, almost all of them have an odd atomic number (meaning an odd number of protons).
The list of stable elements ends after lead. This is partly because nuclei with 128 neutrons are very unstable and quickly undergo alpha decay. This is why elements like astatine, radon, and francium are so short-lived.
What Are Nuclear Isomers?
Among the 251 known stable nuclides, there's a special one called tantalum-180m. This isn't a normal ground state nucleus; it's a nuclear isomer, which means it's an excited (higher energy) state of the tantalum-180 nucleus. The regular tantalum-180 (its ground state) is radioactive and has a half-life of only 8 hours. But tantalum-180m is incredibly stable because its decay is very difficult due to its specific spin and energy. Scientists have observed that its half-life must be more than 10 followed by 15 zeros years! No one has ever seen it decay, so it's considered "observationally stable" and is included in the list of stable nuclides.
Isotopes That Might Decay (But Haven't Yet!)
Scientists believe that as our experimental tools get better, we might discover that some isotopes currently thought to be stable are actually very, very slightly radioactive. For example, in 2003, it was confirmed that bismuth-209 (the only naturally occurring isotope of bismuth) is indeed radioactive. Its half-life is about 20 quintillion (20,000,000,000,000,000,000) years! This is more than a billion times the age of the universe, which is why it was thought to be stable for so long.
Isotopes that are predicted to be unstable but haven't been seen to decay are called observationally stable. Currently, there are 105 such isotopes. Many of these "stable" nuclides are "metastable" in that they could release energy if they decayed. They are expected to undergo very rare types of radioactive decay, like double beta decay.
About 146 nuclides (from elements 1 to 66, except technetium, promethium, samarium, and europium) are theoretically stable to any known type of nuclear decay. This means they are not expected to decay at all, unless something extremely rare like proton decay (which has never been observed) or spontaneous fission happens.
For heavier elements, other theoretical decay paths include:
- alpha decay (where the nucleus spits out an alpha particle)
- double beta decay (where two beta decays happen at once)
- beta decay (where a neutron turns into a proton, or vice versa)
- electron capture (where the nucleus captures an electron)
- double electron capture
- isomeric transition (when an excited nucleus releases energy)
These processes are allowed by physics, but they are very, very rare because of strong "rules" that suppress them.
Summary of Nuclide Types
Here's a quick summary of different types of nuclides. Keep in mind that these numbers can change as scientists learn more!
| Type of Nuclide | Number | Total So Far | Notes |
|---|---|---|---|
| Theoretically stable (not expected to decay by known methods) | 146 | 146 | Includes elements up to 66, except Technetium, Promethium, Samarium, and Europium. |
| Unstable in theory, but decay not yet seen (Observationally Stable) | 105 | 251 | This is the total number of nuclides we currently consider "stable." |
| Radioactive primordial nuclides (long-lived, found naturally) | 35 | 286 | Examples include bismuth, thorium, and uranium. |
| Radioactive non-primordial (made recently on Earth) | ~61 | ~347 | These are made by cosmic rays or are decay products of primordial nuclides (like francium). |
List of Stable Nuclides
Here is a list of stable nuclides. The ones that are italicized are primordial radionuclides (meaning they are radioactive but have been around since Earth formed).
- Hydrogen-1
- Hydrogen-2
- Helium-3
- Helium-4
- no mass number 5
- Lithium-6
- Lithium-7
- no mass number 8
- Beryllium-9
- Boron-10
- Boron-11
- Carbon-12
- Carbon-13
- Nitrogen-14
- Nitrogen-15
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Fluorine-19
- Neon-20
- Neon-21
- Neon-22
- Sodium-23
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Aluminium-27
- Silicon-28
- Silicon-29
- Silicon-30
- Phosphorus-31
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Chlorine-35
- Chlorine-37
- Argon-36
- Argon-38
- Argon-40
- Potassium-39
- Potassium-40 – long-lived primordial radionuclide
- Potassium-41
- Calcium-40
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46
- Calcium-48 – long-lived primordial radionuclide
- Scandium-45
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Vanadium-50 – long-lived primordial radionuclide
- Vanadium-51
- Chromium-50
- Chromium-52
- Chromium-53
- Chromium-54
- Manganese-55
- Iron-54
- Iron-56
- Iron-57
- Iron-58
- Cobalt-59
- Nickel-58
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Copper-63
- Copper-65
- Zinc-64
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Germanium-76 – long-lived primordial radionuclide
- Arsenic-75
- Selenium-74
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80
- Selenium-82 – long-lived primordial radionuclide
- Bromine-79
- Bromine-81
- Krypton-78 – long-lived primordial radionuclide
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86
- Rubidium-85
- Rubidium-87 – long-lived primordial radionuclide
- Strontium-84
- Strontium-86
- Strontium-87
- Strontium-88
- Yttrium-89
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94
- Zirconium-96 – long-lived primordial radionuclide
- Niobium-93
- Molybdenum-92
- Molybdenum-94
- Molybdenum-95
- Molybdenum-96
- Molybdenum-97
- Molybdenum-98
- Molybdenum-100 – long-lived primordial radionuclide
- Technetium – no stable isotopes
- Ruthenium-96
- Ruthenium-98
- Ruthenium-99
- Ruthenium-100
- Ruthenium-101
- Ruthenium-102
- Ruthenium-104
- Rhodium-103
- Palladium-102
- Palladium-104
- Palladium-105
- Palladium-106
- Palladium-108
- Palladium-110
- Silver-107
- Silver-109
- Cadmium-106
- Cadmium-108
- Cadmium-110
- Cadmium-111
- Cadmium-112
- Cadmium-113 – long-lived primordial radionuclide
- Cadmium-114
- Cadmium-116 – long-lived primordial radionuclide
- Indium-113
- Indium-115 – long-lived primordial radionuclide
- Tin-112
- Tin-114
- Tin-115
- Tin-116
- Tin-117
- Tin-118
- Tin-119
- Tin-120
- Tin-122
- Tin-124
- Antimony-121
- Antimony-123
- Tellurium-120
- Tellurium-122
- Tellurium-123
- Tellurium-124
- Tellurium-125
- Tellurium-126
- Tellurium-128 – long-lived primordial radionuclide
- Tellurium-130 – long-lived primordial radionuclide
- Iodine-127
- Xenon-124 – long-lived primordial radionuclide
- Xenon-126
- Xenon-128
- Xenon-129
- Xenon-130
- Xenon-131
- Xenon-132
- Xenon-134
- Xenon-136 – long-lived primordial radionuclide
- Caesium-133
- Barium-130 – long-lived primordial radionuclide
- Barium-132
- Barium-134
- Barium-135
- Barium-136
- Barium-137
- Barium-138
- Lanthanum-138 – long-lived primordial radionuclide
- Lanthanum-139
- Cerium-136
- Cerium-138
- Cerium-140
- Cerium-142
- Praseodymium-141
- Neodymium-142
- Neodymium-143
- Neodymium-144 – long-lived primordial radionuclide
- Neodymium-145
- Neodymium-146
- no mass number 147
- Neodymium-148
- Neodymium-150 – long-lived primordial radionuclide
- Promethium - no stable isotopes
- Samarium-144
- Samarium-146 – probable long-lived primordial radionuclide
- Samarium-147 – long-lived primordial radionuclide
- Samarium-148 – long-lived primordial radionuclide
- Samarium-149
- Samarium-150
- no mass number 151
- Samarium-152
- Samarium-154
- Europium-151 – long-lived primordial radionuclide
- Europium-153
- Gadolinium-152 – long-lived primordial radionuclide
- Gadolinium-154
- Gadolinium-155
- Gadolinium-156
- Gadolinium-157
- Gadolinium-158
- Gadolinium-160
- Terbium-159
- Dysprosium-156
- Dysprosium-158
- Dysprosium-160
- Dysprosium-161
- Dysprosium-162
- Dysprosium-163
- Dysprosium-164
- Holmium-165
- Erbium-162
- Erbium-164
- Erbium-166
- Erbium-167
- Erbium-168
- Erbium-170
- Thulium-169
- Ytterbium-168
- Ytterbium-170
- Ytterbium-171
- Ytterbium-172
- Ytterbium-173
- Ytterbium-174
- Ytterbium-176
- Lutetium-175
- Lutetium-176 – long-lived primordial radionuclide
- Hafnium-174 – long-lived primordial radionuclide
- Hafnium-176
- Hafnium-177
- Hafnium-178
- Hafnium-179
- Hafnium-180
- Tantalum-180m ^
- Tantalum-181
- Tungsten-180 – long-lived primordial radionuclide
- Tungsten-182
- Tungsten-183
- Tungsten-184
- Tungsten-186
- Rhenium-185
- Rhenium-187 – long-lived primordial radionuclide
- Osmium-184 – long-lived primordial radionuclide
- Osmium-186 – long-lived primordial radionuclide
- Osmium-187
- Osmium-188
- Osmium-189
- Osmium-190
- Osmium-192
- Iridium-191
- Iridium-193
- Platinum-190 – long-lived primordial radionuclide
- Platinum-192
- Platinum-194
- Platinum-195
- Platinum-196
- Platinum-198
- Gold-197
- Mercury-196
- Mercury-198
- Mercury-199
- Mercury-200
- Mercury-201
- Mercury-202
- Mercury-204
- Thallium-203
- Thallium-205
- Lead-204
- Lead-206
- Lead-207
- Lead-208
- Bismuth ^^ and above –
- no stable isotopes
- no mass number 209 and above
- Bismuth-209 – long-lived primordial radionuclide
- Thorium-232 – long-lived primordial radionuclide
- Uranium-235 – long-lived primordial radionuclide
- Uranium-238 – long-lived primordial radionuclide
- Plutonium-244 – probable long-lived primordial radionuclide
- Bismuth ^^ and above –
^ Tantalum-180m is a "metastable isotope." This means it's an excited state of tantalum-180. Even though it's an excited state, its half-life is so incredibly long that it has never been seen to decay. This makes it an "observationally stable" primordial nuclide, and it's the only nuclear isomer on this list.
^^ Bismuth-209 was thought to be stable for a long time. This is because its half-life is 2.01 × 1019 years, which is more than a billion times older than the universe itself!
See Also
 In Spanish: Isótopo estable para niños
In Spanish: Isótopo estable para niños
- Isotope geochemistry
- List of elements by stability of isotopes
- List of nuclides
- Mononuclidic element
- Periodic table
- Primordial nuclide
- Radionuclide
- Stable isotope ratio
- Table of nuclides
- Valley of stability
 | May Edward Chinn |
 | Rebecca Cole |
 | Alexa Canady |
 | Dorothy Lavinia Brown |


